L. LERA1, C. ALBALA1, H. SÁNCHEZ1, B. ANGEL1, M.J. HORMAZABAL1, C. MÁRQUEZ1, P. ARROYO2
1. Institute of Nutrition and Food Technology (INTA) – University of Chile, Santiago, Chile; 2. Radiology Department, Clinical Hospital, University of Chile, Independencia, Santiago, Chile.
Corresponding author: Dr. Cecilia Albala, Public Health Nutrition Unit, Institute of Nutrition and Food Technology (INTA) – University of Chile, El Líbano 5524, Casilla 138-11, Santiago, Chile, E-mail: calbala@uchile.cl
J Frailty Aging 2017;in press
Published online November 30, 2016, http://dx.doi.org/10.14283/jfa.2016.117
Abstract
Abstract: Background: Sarcopenia is the progressive loss of mass and skeletal muscle strength and has serious consequences on older people’s health. The Chilean older population has a high life-expectancy, but the prevalence of functional dependence is also high. Objective: To determine the prevalence of sarcopenia in Chilean older adults and its relationship with age, gender, and body mass index (BMI). Design: Cross-sectional study. Setting: Community. Participants: 1,006 non-disabled, community-dwelling subjects aged 60 years or older living in Santiago. Measurements: Anthropometric measurements, handgrip strength, physical performance tests, and dual-energy-x-ray-absorptiometry (DXA) scan were performed. Sarcopenia was defined using the algorithm of the European Working Group on Sarcopenia in Older People (EWGSOP). Muscle mass was measured with DXA scan; skeletal muscle mass index (SMI) and hand dynamometry were defined with cut-off points obtained for the Chilean population. For a 3m walking speed we used the cut-off point of the EWGSOP definition. Nutritional status and obesity were defined according to World Health Organization standards. Association between sarcopenia and age, gender, BMI and lean/fat mass ratio was estimated by logistic regression models. Results: The prevalence of sarcopenia was 19.1% (95%CI: 16.8%-21.8%), similar in men and women. There was an increasing trend of sarcopenia by age group and a decreasing trend with nutritional status. After logistic regression, sarcopenia was positively associated with age (OR=1.10; 95%CI:1.06-1.15) and falls (OR=1.83; 95%CI:1.07-3.15) and negatively associated with overweight (OR=0.31; 95%CI:0.16-0.59), obesity (OR=0.02; 95%CI:0.004-0.11), lean mass/fat mass ratio (OR=0.69; 95%CI:0.48-0.9997), knee height (OR=0.78; 95%CI:0.68-0.89) and calf circumference (OR=0.87; 95%CI:0.77-0.97). Conclusions: The total prevalence of sarcopenia was 19.1% increasing with age reaching 39.6% in people of 80 or more years of age. A negative association of sarcopenia with overweight, obesity and lean/fat mass ratio was observed. Although the high prevalence of obesity (35.9%), only 2% of obese people were sarcopenic.
Key words: Sarcopenia, muscle mass, muscle strength, community-dwelling older people.
Introduction
Sarcopenia is a characteristic of biological ageing that involves a progressive loss of mass and skeletal muscle strength and has a serious impact on the health of older people (1–3). Considering that sarcopenia is mainly an age-associated syndrome, in fast aging countries with large socioeconomic inequalities such as Latin American countries, the adverse consequences of this syndrome can be immense. Chile has a life expectancy at birth comparable to the developed world, reaching 82.2 for women and 78.5 for men in the period 2010–2015. However, the prevalence of functional dependence is also high, mainly in people of low socioeconomic status (4).
The prevalence of sarcopenia varies according to age group and the criteria used. The wide variation reported ranged from 13.5% to 25% in people under 70 years and from 25% to 60% in people over 80 years of age (5,6) and is probably due to a lack of specific cut-off points for population from different ethnic backgrounds and the different techniques used for assessing muscle mass. Muscle mass can be measured with a dual-energy x-ray absorptiometry (DXA) scan, magnetic resonance imaging or Bioimpedance analysis (1). A DXA scan is the most commonly used as gold standard (7).
In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) developed a practical clinical definition and consensus diagnostic criteria by means of a diagnostic algorithm. The EWGSOP defines sarcopenia as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death” (1).
The diagnostic algorithm is based on a screening in series where muscle function (physical performance and strength muscle) is evaluated first. If the subject has low muscle function, then it is necessary to evaluate body composition, in particular appendicular skeletal muscle mass (ASM) (1). Using the definition of the EWGSOP, the recently published review (6) reported a prevalence of sarcopenia varying between 1% and 30%. Considering that the racial, ethnic composition, and morphological characteristics of the Latin American population differs from the European (8), we used cut-off points of muscle mass measured by DXA scan and muscle strength obtained in a large Chilean population (9). The objective of this study was to calculate the prevalence of sarcopenia and its associated factors in Chilean older people using the algorithm of the EWGSOP.
Methods
Cross-sectional study in 1,006 (68.3% female) community-dwelling, non-disabled subjects ≥60 years living in Santiago and participating in ALEXANDROS and ISAmayor studies (4,10).
After signing an informed consent approved by the ethics committee of the Institute of Nutrition and Food Technology, all subjects underwent face-to-face interviews including self-reported chronic diseases (hypertension, diabetes, cancer, COPD, stroke), self-reported functional limitations, and self-perceived symptoms of depression measured by the Short Form of the Geriatric Depression Scale (GDS-15).
A DXA scan was performed in the whole sample in order to assess body composition. Handgrip strength was measured by means of handgrip dynamometry (Hand Dynamometer T-18, Country Technology, Inc.), registering the best of two measurements with the dominant hand. Three meters walking speed was registered. Anthropometric measurements of weight, height, and knee height; as well as waist, hip, calf, and arm circumferences were done according to methods described previously (10). Their Appendicular skeletal muscle mass index (SMI) was calculated as the ratio of ASM and height2 (kg/m2). Low muscle mass was defined using the 20th percentile (p20) of SMI calculated on a sample of 565 subjects (9). Nutritional status and obesity were defined according to World Health Organization (WHO) standards. Sarcopenia was defined using the consensus criteria and the algorithm of the EWGSOP (1). Low SMI was defined with cut-off points obtained for the Chilean population (men: 0.8 m/sec).
Stages of sarcopenia —pre-sarcopenia, sarcopenia, and severe sarcopenia— were also determined by the suggested classification of the EWGSOP (1). These are characterized by low muscle mass (pre-sarcopenia), low muscle mass and low muscle strength or low physical performance (sarcopenia) and low muscle mass, low muscle strength and low physical performance (severe sarcopenia).
Statistical analysis
Continuous variables were expressed as mean ± Standard Deviation (SD) and 95% confidence intervals (95%CI). Categorical variables were expressed as median or percentages and 95%CI. The difference between genders was calculated by a two-sample mean-comparison test or Pearson’s Chi2 test, depending on the kind of variable. Differences among age groups and levels of sarcopenia were estimated by Pearson‘s Chi2 test and by a test for trend across ordered groups. Logistic regression models were performed to analyze the association between studied variables (risk factors, age, anthropometric measurements), adjusted by sex and the presence or absence of sarcopenia. The Hosmer–Lemeshow test was used to assess the goodness of fit for the estimated models. The ratio of lean mass to fat mass was calculated to adjust the relationship between BMI and sarcopenia.
All statistical analyses were performed using STATA 14 (StataCorp.2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Table 1 shows the socio-demographic, health characteristics, anthropometric variables, body composition, handgrip strength, and physical performance tests of the study sample by sex. Men represented 31.7% of the total sample. The mean age was similar for men and women (67.7±5.7 and 67.6±6.0 years; respectively). There were no differences between age groups by gender. Less than 11% of the older adults lived alone: 8.8% of the men and 10.2% of the women lived alone (non-significant). The mean years of education were similar in men and women (7.5 and 7.7 years; respectively), and the distribution of the different levels was also similar by sex. The frequency of one or more disabilities on the Activities of Daily Living (ADL) was similar in men and women (4.7% vs 3.9%; respectively), and the percentage of one or more disabilities on the Instrumental Activities of Daily Living (IADL) was significantly higher in men than women (17.6% vs 12.9%). Diabetes and symptoms of depression were similar in men and women. The percentage of men with zero diseases was significantly higher than the women’s (39.4% vs 31.6%; respectively). Men smoked significantly more than women (19.1% vs 13.1%; respectively). The number of falls and fractures were significantly higher in women than men (Falls: 37.8% vs 22.9%; Fractures: 18.9% vs 12.9%; respectively). The BMI was higher in women than men (29.2 kg/m2 vs 27.8
kg/m2; respectively), and consequently the prevalence of obesity (39.8% vs 27.7%; respectively). Lower handgrip strength (18.7 kg vs 32.8 kg), ASM (14.4 kg vs 21 kg), SMI (6.3 kg vs 7.7 kg), lean mass (35.8 kg vs 49.7 kg) and lean mass/fat mass ratio (1.4 vs 2.5) were found to be lower in women than in men. Fat mass was higher in women than men (28.5 kg vs 23.7 kg; respectively). With regard to the physical performance test (3 meters walking speed), men had higher values than women (0.89 m/s vs 0.80 m/s; respectively), with 68.2% of the men and 49.2% of women achieving a speed >0.8 m/sec.
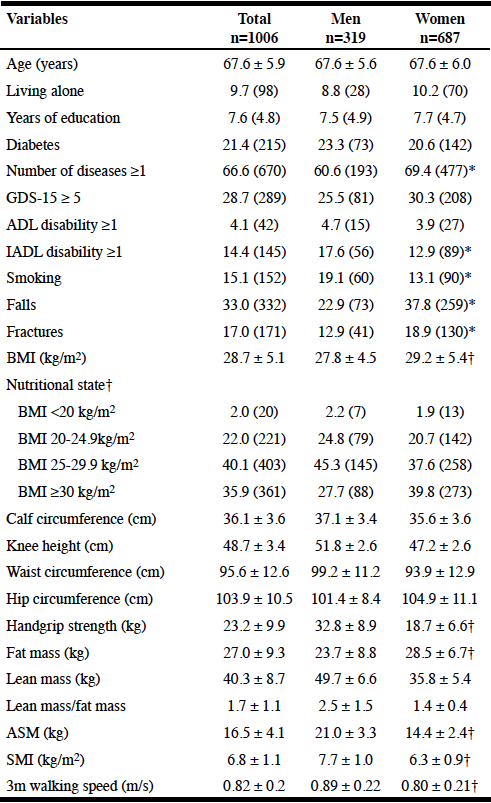
Table 1
Socio-demographic, health characteristics and physical performance of the study sample by sex
Results are presented as mean ± SD, or percentage (n);GDS: Geriatric depression scale; ADL: Activities of Daily Living; IADL: Instrumental ADL; BMI: Body Mass Index: ASM: Appendicular Skeletal Muscle: SMI: Skeletal Muscle Index; * p<0.05; † p<0.0001
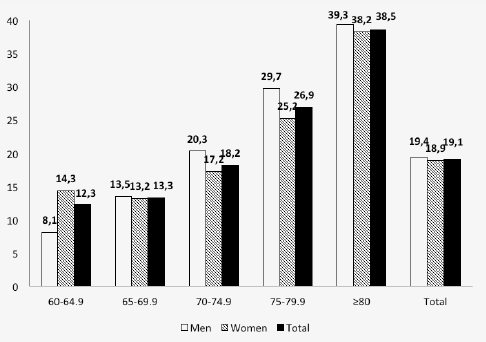
The prevalence of sarcopenia by age group (60-64.9; 65-69.9; 70-74.9; 75-79.9 and ≥80 years), gender, and whole sample is shown in Figure 1. The prevalence increases significantly with increasing age groups in the total sample and by gender. The prevalence of sarcopenia in the total sample is similar in men and women (19.4% vs 18.9%; respectively).
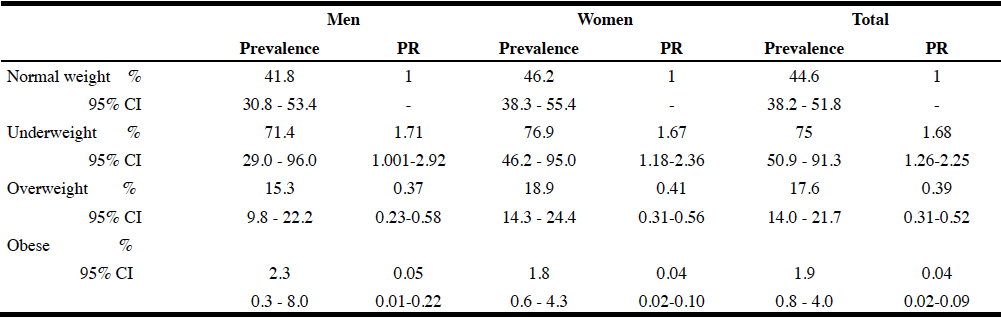
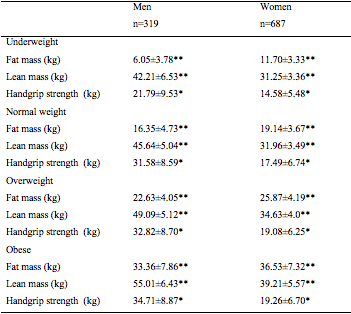
The prevalence and the prevalence ratio of sarcopenia by nutritional status and gender are presented in Table 2. There is a decreasing trend of sarcopenia with nutritional status in both genders (men: 71.4; 41.8; 15.3; 2.3% and women: 76.9; 46.8; 19; 1.9%), showing only 2% of the obese being sarcopenic. We observed a dose-response relationship in relation to nutritional status; older people with low weight (BMI<20 kg/m2) are 1.7 times more likely to be sarcopenic than people presenting a normal weight, whereas overweight and obese adults (BMI≥25 kg/m2) are less likely to be sarcopenic compared to subjects of normal weight for the entire sample and by gender. Overweight and obese subjects had higher lean mass and grip strength than underweight and normal subjects (see supplementary table).
Reference: Normal weight; CI: confidence interval; PR: Prevalence Ratio; p<0.0001 (Sarcopenia vs Nutritional status by gender)
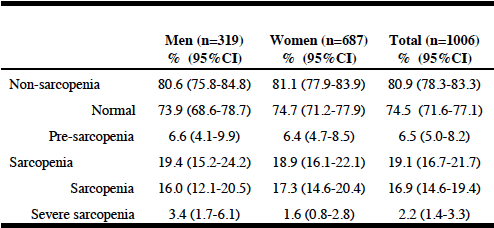
Table 3 shows the classification of stages of sarcopenia by gender. Pre-sarcopenia was identified in 6.5% of the total sample and severe sarcopenia in 2.2% of the sample. No differences between men and women were observed.
Three logistic regression models for the association of sarcopenia with anthropometric variables, lean/fat mass ratio, diabetes and falls adjusted by age and sex are displayed in Table 4. In an age, sex and lean/fat mass ratio adjusted model, compared with those with normal nutritional state, overweight (OR=0.21; 95%CI: 0.13-0.30) and obesity (OR=0.017; 95%CI: 0.008-0.04) were independently associated with lower risk of sarcopenia. On the other hand underweight was associated with higher risk of sarcopenia (OR=9.10; 95%CI: 2.16-38.32). After additional adjustment by knee height, calf circumference, diabetes and falls the negative association of obesity (OR=0.31; 95%CI: 0.16-0.59) and overweight (OR=0.02; 95%CI: 0.004-0.11) remained significant. On the other hand, a positive association of sarcopenia with falls was observed (OR=1.83; 95%CI:1.07-3.15).
Pearson‘s Chi2 test: Sarcopenia levels vs gender: p>0.1
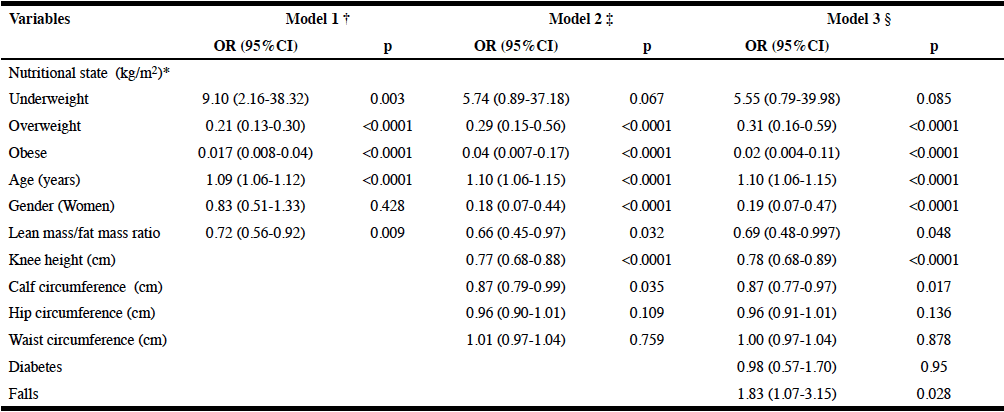
Table 4
Logistic regression models of the risk factors associated with sarcopenia, adjusted by sex and age
OR: odds ratio; CI: confidence interval; Reference category of dependent variable: Non-sarcopenia; * Normal nutritional state is used as reference group; Hosmer-Lemeshow’s goodness-of-fit test: † Chi2(8) =9.75; p=0.28; ‡ Chi2(8) =3.64; p=0.89; § Chi2(8) =7.06; p=0.53
Discussion
In this study, we report the prevalence of sarcopenia in a sample of 1,006 older adults Chileans using the consensus definition developed by the EWGSOP (1), which was previously validated by our group (9). We found a prevalence of sarcopenia of 19.1%, similar for men and women. The prevalence of sarcopenia increased with age from 12.3% in the 60–64 y group to 38.5% in subjects ≥80 years. A wide range of sarcopenia prevalence (4.1 to 24.2) has been reported in Europe, Asia, and the USA with the same diagnostic criteria (1) (1, 6, 7, 12–15). In Asian countries, the figures vary in different studies. The study of Yamada in community-dwelling Japanese people aged 65 to 89 found a slightly higher prevalence of sarcopenia than ours: 21.8% in men and 22.1% in women (16). However, Sanada, using two reference cut-off points for SMI from Japanese people, found a prevalence between 6.7% and 56.7% in men and 6.3% and 33.6% in women aging 70-85 years, depending on the reference values of SMI used (17).
In Taiwan, Wu et al. (18) studied the prevalence of sarcopenia and associated factors with severe sarcopenia in 549 older adults living in communities situated in both urban and rural zones using the consensus definition developed by the EWGSOP. They found that only 12.7% of the subjects had sarcopenia. However, it is important to highlight that differences may be due to different cut-off points or methods used, even though the same diagnostic criteria was employed. Recently, in a Belgian study (19) conducted in 400 people ≥65 years of age, the prevalence of sarcopenia varied between 9.25% and 18% depending on the different cut-offs and criteria used for diagnosing sarcopenia.
On the other hand, in Taiwan (20) the prevalence of sarcopenia was found to vary between 5.8% to 14.9% in men and 4.1% to 16.6% in women, depending on the International Working Group on Sarcopenia or the EWGSOP criteria used.
Studies are scarce in Latin America. To our knowledge, this is the first study in Latin America calculating the prevalence of sarcopenia using a DXA scan to measure ASM. A study of prevalence done in Mexico City estimated the prevalence of sarcopenia in 25.6%, in the group of 60 to 79 years of age and 50.4% in subjects ≥80 years of age. In that study, lower muscle mass was estimated using a calf circumference lower than 31 cm (21), a good clinical indicator of disability and physical function, but not as DXA for screening of sarcopenia (2). A similar study, using calf circumference, estimated prevalence of sarcopenia (11.5%) and frailty (9.4%) in Colombia (22). In a Brazilian study, muscle mass was estimated through the Lee prediction equation. They found a prevalence of sarcopenia of 16.1% in women and 14.4% in men from the SABE study (23) using the EWGSOP diagnostic algorithm.
In relation to the different stages of sarcopenia, only 2.2% of the sample in the current study had severe sarcopenia. We found only one study reporting severe sarcopenia using the EWGSOP definition in the Hertfordshire Cohort Study (24) with results similar to ours (men 1.9%; women 2%). The published prevalence of severe sarcopenia are based on different definitions and methods to assess muscle mass. Janssen, in the Third National Health and Nutrition Examination Survey using Bioelectrical Impedance, found prevalence ranging from 7 to 10% depending on age, with the definition of being under 2SD of young adults’ SMI. Similar results with the same methods were found in some Asian studies (25).
With respect to factors associated with sarcopenia, our main finding is the negative association with BMI (18), with only 2% of the obese being sarcopenic. Moreover, a dose-response was observed in the prevalence of sarcopenia according to nutritional status, showing a negative gradient from underweight to obesity. The prevalence of obesity in the studied group was high (35.9%), although the obesity defined by a BMI≥30kg/m2 was mainly non-sarcopenic. However, in older people, the single use of BMI as a proxy for body composition can be misleading as the same BMI can correspond to different proportions of fat mass and lean mass. To avoid biased interpretations of the former association, we performed a multivariable analysis adjusting by the ratio of lean to fat mass and knee height, a measure that is more representative of the skeleton size in older people (26,27) than the current height. After adjusting, the association remains invariable, meaning that in these cohorts, obese people are not sarcopenic. Obesity has been consistently associated with longer survival (28) and mild obesity in older people has shown a paradoxical association with lower morbidity and better evolution of some chronic diseases such as heart failure and osteoporosis (29). However, the huge lifestyle changes of the Chilean population that have occurred in the past three decades, with high prevalence of obesity and sedentary behaviour in all groups of age, will lead to a future aged population more vulnerable to sarcopenic obesity. Other studies in younger cohorts are needed to assess the effect of demographic origin on that characteristic.
Like Morley (13), we also found a strong association between sarcopenia and falls within the past year.
The main limitation of the study is that the cross-sectional design does not allow us to provide evidence about risk factors or consequences of sarcopenia. This issue will be addressed with the follow-up of the subjects involved in the study. Among the strengths of this study, the most important one is that the prevalence of sarcopenia was estimated in a large sample of people based on DXA scan measurements. This type of measurement is one of the most recommended imaging techniques considering its accuracy, reproducibility and sensitivity (30), although its accessibility in countries with low or medium low economies is scarce. In addition, we used cut-off points of SMI and muscle strength obtained for the Chilean population (9). This will allow us to make international comparisons with similar techniques.
Since sarcopenia is an important cause of multiple negative health outcomes in older people, knowing its actual magnitude is crucial for developing policies and programs for its prevention and control.
Funding: This project was supported by FONIS SA12I2337 (Fondo Nacional de Investigación y Desarrollo en Salud) and FONDECYT 1130947 (Fondo Nacional de Desarrollo Científico y Tecnológico).
Conflict of interest: None
References
1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23.
2. Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 2008;12:433–50.
3. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S – 991S.
4. Albala C, Sánchez H, Lera L, Angel B, Cea X. [Socioeconomic inequalities in active life expectancy and disability related to obesity among older people]. Rev Med Chil 2011;139:1276–85.
5. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63.
6. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014; 43:748-59.
7. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1:129–33.
8. Sol M del, Vásquez B, Cantín M. Características Morfológicas Métricas y No Métricas del Esternón del Individuo Mapuche. Int J Morphol 2014;32:351–6.
9. Lera L, Ángel B, Sánchez H, et al. [Validation of cut points of skeletal muscle mass index for identifying sarcopenia in Chilean older people]. Nutr Hosp 2015;31:1187–97.
10. Lera L, Albala C, Ángel B, et al. [Anthropometric model for the prediction of appendicular skeletal muscle mass in Chilean older adults]. Nutr Hosp 2014;29:611–7.
11. Arroyo P, Lera L, Sánchez H, Bunout D, Santos JL, Albala C. [Anthropometry, body composition and functional limitations in the elderly]. Rev Med Chil 2007;135:846–54.
12. Morley JE. Sarcopenia in the elderly. Fam Pract 2012;29:i44–8.
13. Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle 2014;5:253–9.
14. Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 2002;57:M772–7.
15. Tichet J, Vol S, Goxe D, Salle A, Berrut G, Ritz P. Prevalence of sarcopenia in the French senior population. J Nutr Heal Aging 2008;12:202–6.
16. Yamada M, Nishiguchi S, Fukutani N, et al. Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc 2013;14:911–5.
17. Sanada K, Miyachi M, Tanimoto M, et al. A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol 2010;110:57–65.
18. Wu CH, Chen KT, Hou MT, et al. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: The Tianliao Old People study 04. Geriatr Gerontol Int 2014;14:69–75.
19. Beaudart C, Reginster J, Slomian J, Buckinx F, Locquet M, Bruyère O. Prevalence of sarcopenia : the impact of different diagnostic cut-off limits. J Musculoskelet Neuronal Interact 2014;14:425–31.
20. Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK. Comparisons of Sarcopenia Defined by IWGS and EWGSOP Criteria Among Older People: Results From the I-Lan Longitudinal Aging Study. J Am Med Dir Assoc 2013;14: 528.e1–7
21. Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU. Prevalence of sarcopenia in Mexico City. Eur Geriatr Med 2012;3:157–60.
22. Samper-Ternent R, Reyes-Ortiz C, Ottenbacher KJ, Cano CA. Frailty and sarcopenia in Bogotá: results from the SABE Bogotá Study. Aging Clin Exp Res 2016. http://dx.doi.org/10.1007/s40520-016-0561-2.
23. Alexandre T da S, Duarte YA de O, Santos JLF, Wong R, Lebrão ML. Prevalence and associated factors of sarcopenia among elderly in Brazil: findings from the SABE study. J Nutr Health Aging 2014;18:284–90.
24. Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013;42:378–84.
25. Abe T, Thiebaud RS, Loenneke JP, Loftin M, Fukunaga T. Prevalence of site-specific thigh sarcopenia in Japanese men and women. Age 2014;36:417–26.
26. Chumlea WC, Roche AF, Steinbaugh ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc 1985;33:116–20.
27. Lera L, Luis Santos J, García C, Arroyo P, Albala C. Predictive equations for stature in the elderly: a study in three Latin American cities. Ann Hum Biol 2005;32:773–81.
28. Rolland Y, Gallini A, Cristini C, et al. Body-composition predictors of mortality in women aged ≥ 75 y: data from a large population-based cohort study with a 17-y follow-up. Am J Clin Nutr 2014;100:1352–60.
29. Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: Facts and numbers. J Cachexia Sarcopenia Muscle 2012;3:1–4.
30. Cesari M, Fielding RA, Pahor M, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 2012;3:181–90.
1Test for trend across ordered groups: *p<0.02; **p<0.0001