F. Landi1, C. Sieber2, R.A. Fielding3, Y. Rolland4, J. Guralnik5 and the ICFSR Task Force
1. Fondazione Policlinico A. Gemelli, Catholic University, Rome, Italy; 2. Institut für Biomedizin des Alterns, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nürnberg, Germany; 3. Nutrition, Exercise Physiology, and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA; 4. Service de Médecine Interne et Gérontologie, Clinique Gérontopôle, Hôpital La Crave, Casselardit, Toulouse, France; 5. University of Maryland School of Medicine, Baltimore, MD, USA
Corresponding author: Francesco Landi, Fondazione Policlinico A. Gemelli, Catholic University, Rome, Italy, francesco.landi@unicatt.it
Task Force members: Hidenori Arai (Obu City, Japan); Mylène Aubertin-Leheudre (Montréal, Canada); Jürgen Bauer (Heidelberg, Germany); Ryne Carney (Washington, USA); Brian Clark (Athens, USA); Alfonso Cruz Jentoft (Madrid, Spain); Carla Delannoy (Vevey, Switzerland); Susanna Del Signore (Paris, France); Elsa Dent (Adelaide, Australia); Waly Dioh (Paris, France); Roger Fielding (Boston, USA); Bertrand Fougère (St Louis, USA); Juerg Gasser (Basel, Switzerland); Jack Guralnik (Baltimore, USA); Hare Joshua (Miami, USA); Aaron Hinken (King of Prussia, USA); Evgueni Ivanov (Basel, Switzerland); Naotoshi Kanemitsu (Tokyo, Japan); Kala Kaspar (Vevey, Switzerland); Tatiana Klompenhouwer (Utrecht, The Netherlands); Stephen Kritchevsky (Winston-Salem, USA); Francesco Landi (Roma, Italy); Valérie Legrand (Nanterre, France); Yvette Luiking (Utrecht, The Netherlands); Ram Miller (Cambridge, USA); Bradley Morgan (South San Francisco, USA); John Morley (St Louis, USA); Vikkie Mustad (Columbus, USA); David Neil (King of Prussia, USA); Suzanne Page (Miami, USA); Marco Pahor (Gainesville, USA); Dimitris Papanicolaou (East Hanover, USA); Suzette Pereira (Columbus, USA); Claire Regard (Vevey, Switzerland); Daniel Rooks (Cambridge, USA); Jorge Ruiz (Miami, USA); Cornel Sieber (Nürnberg, Germany); Sitra Tauscher Wisniewski (Northbrook, USA); Brooke Travnicek (Clearwater, USA); Vellas Bruno (Toulouse, France); Dennis Villareal (Houston, USA); Debra Waters (Dunedin,New Zealand); Lixin Zhang Auberson (Basel, Switzerland)
J Frailty Aging 2018;7(4):247-252
Published online September 20, 2018, http://dx.doi.org/10.14283/jfa.2018.26
Abstract
Research suggests that poor nutrition is an underlying cause of sarcopenia and frailty, and that dietary interventions may prevent or treat age-related loss of muscle mass and strength. In February 2018, the International Conference on Frailty and Sarcopenia Research Task Force explored the current status of research on nutritional interventions for sarcopenia as well as gaps in knowledge, including whether nutritional supplements must be combined with physical activity, and the role of nutritional intervention in sarcopenic obese individuals. The lack of consistency across trials in terms of target populations, assessments, health-care settings, control groups, and choice of outcomes has made it difficult to draw meaningful conclusions from recent studies. The Task Force recommended large randomized controlled trials in heterogeneous, real-world populations to enable sub-group analysis. The field also needs to reach consensus on what outcomes are most meaningful and what represents clinically meaningful change.
Key words: Sarcopenia, nutritional intervention, physical activity, clinical trials, protein supplementation.
Introduction
Sarcopenia, the age-related loss of muscle mass and strength, is recognized as a substantial contributor to disability and loss of independence in older individuals, as well as a major public health problem (1). Nonetheless, interventions for sarcopenia are sparse, owing in part to a lack of consensus on definitions and an insufficient number of well-designed studies (2, 3). Progress in developing treatments for sarcopenia was advanced in 2016 when a unique ICD-10 code was established for sarcopenia as a first step towards recognizing sarcopenia as a reportable condition and enabling the collection of more rigorous data on its biological underpinnings, clinical characteristics, prevalence, impact on function, response to intervention, and other aspects (4). Yet even as the pharmaceutical industry advances several different classes of drugs that may attenuate or reverse muscle atrophy (3), non-pharmaceutical interventions such as nutritional and physical activity approaches have emerged as having both therapeutic and preventive benefits (5).
In February 2018, the International Conference on Frailty and Sarcopenia Research Task Force met to discuss the role of poor nutrition as an underlying cause of sarcopenia and the potential for developing nutritional interventions to treat or prevent the condition, whether caused by poor nutrition or other problems. Task Force members noted that nutrition interacts with sarcopenia through biological, psychosocial, and lifestyle mechanisms, yet also recognized that it may be easier for clinicians to prescribe a drug than to get patients to change their lifestyle and that the severity of sarcopenia may dictate whether nutritional, physical activity or pharmacologic approaches are warranted and feasible. Thus, the Task Force concluded that nutrition would need to be integrated with pharmacotherapy and physical activity in order to have a substantial impact on individual patients and the overall prevalence of sarcopenia.
Rationale for nutritional intervention in sarcopenia
Epidemiological studies indicate that chronic undernutrition (inadequate intake of protein and energy; micronutrient deficiencies) contributes to both sarcopenia and frailty. The Third National Health and Nutrition Survey (NHANES III), a population-based cohort study, found that among older adults with sarcopenia, poor diet quality and physical inactivity were associated with a higher risk of mortality (6). Similarly, the Health ABC study of well-functioning, community-dwelling older adults found that low protein intake was associated with increased risk of mobility limitation (7). More recently, results from the Very Important Protein (VIP) survey in Italy suggest an association between protein intake and muscle mass and strength across ages (8).
Indeed, there is a linear relationship between nutritional status and functionality (9). A role for nutrition in the development of frailty was suggested by a pilot study of older adults seen by general practitioners in Germany. Two-thirds of patients in this cohort were determined to be frail or pre-frail, with 30% of frail and 15% of pre-frail individuals experiencing weight loss (10).
Diet quality is related to both sarcopenia and frailty, and nutritional intervention may be able to reduce their incidence (11). Different nutritional approaches have been studied, including supplemental protein, essential amino acids (EAAs), and oral nutritional supplements that combine protein, leucine, the leucine metabolite beta-hydroxy-beta-methylbutyrate (HMB), and vitamins (12). The rationale for supplementing protein in older adults is particularly strong. Older adults have higher protein requirements than do younger adults to maintain good health and functionality and enable recovery from illness (13). Multiple factors contribute to the higher protein needs of older adults, including their reduced anabolic response to protein intake and their elevated prevalence of inflammatory and catabolic conditions associated with aging (14, 15). Yet older adults tend to consume less protein in comparison to young adults (16).
In 2013, an international study group (PROT-AGE) established by the European Union Geriatric Medicine Society (EUGMS) and other scientific organizations published recommendations for dietary protein intake in healthy older adults. The study group concluded that older people not only need more protein than do younger adults, but that specific nutritional strategies – i.e., the protein source, addition of specific amino acids and other nutritional components, timing of intake, and incorporation of an exercise program — need to be adopted to ensure optimal protein digestion and absorption (13).
Some studies have suggested that protein supplements may lead to a reduction in total energy intake (17). This may potentially be mitigated by supplementation of EAAs rather than by adding high-protein supplements to the diet (18). EAAs have been shown to stimulate muscle protein synthesis (19), although this effect may be blunted in older age (20). Leucine and/or HMB supplementation have also been proposed as an effective approach to improve strength and muscle mass in older adults due to their effects on muscle protein synthesis, yet the studies have been somewhat inconsistent (21, 22).
Evidence from past clinical studies
Although not widely studied and appreciated, accumulating evidence indicates that poor skeletal muscle performance is critical in many diseases and conditions including sarcopenia, frailty, orthopedic disuse, muscle atrophy resulting from prolonged bed rest or immobility, neuromuscular diseases, and cachexia from cancer or other conditions (23). Nutrition and dietary studies have examined the role of protein, essential amino acids, leucine and the leucine metabolite HMB, citrulline, and omega-3 fatty acids with and without physical activity on muscle strength and function. Meanwhile, new drugs targeting sarcopenia are emerging from pharmaceutical companies, including compounds that affect muscle growth, neuromuscular contractile properties, and activators of mitochondrial biogenesis. Medicines approved for other conditions are also being studied. Combining pharmaceutical and lifestyle approaches will likely be needed to achieve necessary levels of effectiveness; however, much more research is needed to understand how nutrition interacts with drugs and other lifestyle interventions.
Marginal protein intake has been associated with loss of muscle mass in older adults (24), which increases the risk of developing sarcopenia and other chronic conditions that can lead to disability. In a 2017 meta-analysis of eight randomized clinical trials testing the effect of protein or amino acid supplementation on muscle mass and strength in healthy older adults, Tieland and colleagues found no evidence of a positive effect from either protein or amino acid supplementation on lean body mass, leg press strength, leg extension strength, or handgrip strength (25). Their conclusion suggested that these interventions may require concomitant nutritional or exercise intervention. However, other studies reached different conclusions. For example, the PROVIDE study in sarcopenic older adults showed that while a 13-week intervention with a specific oral nutritional supplement consisting of a leucine-enriched protein and vitamin D but no physical activity component did not improve the primary outcomes — handgrip strength and score on the Short Physical Performance Battery (SPPB) — it did result in a significant improvement in chair-stand time and appendicular muscle mass assessed with DXA (26). Moreover, another study with 6-month duration testing a nutritional supplement containing whey protein and vitamins D and E demonstrated significant improvement in measures of muscle mass, muscle strength, and anabolic markers such as IGF-1 and IL-2 in sarcopenic older adults (27).
Another study with a six-month duration, VIVE-2, tested a combined intervention of physical activity with or without a daily nutritional supplement of whey protein and vitamin D in mobility-limited older adults. This study showed no significant difference in walking speed or SPPB using combined nutritional and physical activity approach (28). However, a significant reduction in intramuscular fat and increase in normal density muscle was observed with the nutritional supplement (29, 30).
Sarcopenia and functional decline are exacerbated with prolonged inactivity, especially in the elderly. For example, healthy elderly lost 9% of leg mass after only 10 days of bed rest (31), while young people lost only 2% of leg mass after 28 days of bed rest. In a study of older individuals restricted to bed rest for 10 days, Ferrando and colleagues showed that increasing protein intake by EAA supplementation appeared to help restore protein metabolism and synthesis, and degradation flux (32). This study supported calls for a higher recommended daily allowance of protein in older adults who are inactive.
Older adults may also have sarcopenia in combination with obesity, although studies report varying prevalence of sarcopenic obesity depending on the definition of sarcopenia used (33). Nutritional approaches to treating sarcopenic obesity must achieve a balance of gaining muscle mass while reducing fat mass. The few studies that have been done in this population suggest that complex interventions are required, which include, in addition to nutritional supplements, a focus on weight management and exercise (34).
Across these various populations, studies have confirmed a link between frailty, sarcopenia, and malnutrition, and provided a rationale for protein and nutritional intervention in sarcopenia. However, many questions remain. For example, it is unclear whether there is a role for a nutritional supplement alone or if it must be combined with physical activity; and the type of physical activity program is as complicated to define as the type of nutritional supplement. More research is also needed to better understand the role of nutritional intervention in sarcopenic obese older person. Many questions also remain about the appropriate time course for trials of nutritional interventions in sarcopenia. When trying to change distal outcomes such as walking ability, stair climbing, or chair rise time with a nutritional intervention in comparison to a drug or even an exercise program, the effects may be subtle and take longer than a typical 6-month trial.
Designing studies with nutritional interventions against sarcopenia
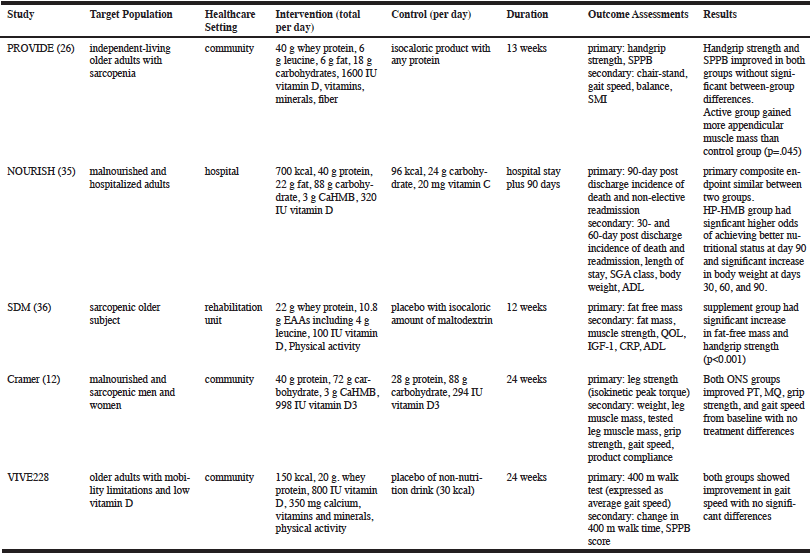
Inconsistencies among recent nutritional interventions studies in sarcopenia make it difficult to integrate their findings into a consistent message about the efficacy of nutritional approaches. Table 1 summarizes five of these recent studies (12, 26, 28, 35, 36), comparing some of the key trial design features as well as the results. The table highlights the lack of consistency among studies in terms of target population, assessments, health-care settings, control group, duration, and choice of primary and secondary outcomes, making it difficult to draw conclusions about the relative merits of the different interventions. Only the NOURISH study included a cost-effectiveness analysis, although this represents an important aspect of any intervention for payers, clinicians, and patients (37).
Target population, healthcare setting, and inclusion/exclusion criteria. All five studies enrolled older adults, either ≥65 years (12, 26, 35, 36) or ≥70 years (28). Participants were enrolled alternatively from community settings (12, 26, 28), rehabilitation units (36), or hospitals (35), and in a single country versus multiple countries. In addition, the inclusion and exclusion criteria varied widely. For example, the PROVIDE Study enrolled sarcopenic, primarily independent-living, non-malnourished older adults with SPPB scores between 4 and 9 and low skeletal muscle mass index (26); while the NOURISH Study enrolled malnourished Subjective Global Assessment (SGA) class B or C adults hospitalized for heart failure, acute myocardial infarction, pneumonia, or chronic obstructive pulmonary disease (35); and the VIVE2 study enrolled older adults with functional limitations but who were able to walk 400 meters in less than 15 minutes and had vitamin D insufficiency (28). Three of the studies excluded individuals with low Mini-Mental State Examination (MMSE) scores, yet the cut-offs varied from <20 to <25. Exclusions for various comorbid conditions also varied substantially among studies.
Types of assessment
Studies used varying functional assessments such as SPPB, hand-grip strength, gait speed, and different measures of leg extension strength and power. Measures of muscle quality also varied substantially. PROVIDE, for example used bioelectric impedance analysis to determine skeletal muscle index, while SDM, Cramer, and VIVE2 used DXA to measure alternatively relative muscle mass (36), skeletal mass index (12), or whole-body and regional fat and muscle mass (30). Even DXA measurement varied among studies, which used either lunar and Hologic machines (38).
Type and duration of intervention
Table 1 summarizes the interventions tested in each study as well as the composition of the placebo provided to the control group. Trial duration ranged from 12 weeks to 24 weeks with the exception of the NOURISH study, which lasted for the length of the hospital stay plus 90 days.
Outcomes
Results of the studies are also summarized in Table 1. The primary endpoint was met only in the SDM study, where supplementation with protein, EAAs, and Vitamin D resulted in a significant increase in fat-free mass compared to the control group that received placebo with an isocaloric amount of maltodextrin. Handgrip strength was also significantly increased in the supplement group. Participants in most of the studies improved regardless of whether they received the active versus control intervention.
Going forward
Applying the lessons learned from these and other previous studies, including the landmark Lifestyle Interventions and Independence For Elders (LIFE) study (39), the Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) study was launched under the umbrella of the Innovative Medicines Initiative-Joint Undertaking (IMI-JU 11561) in 2014, and has recently completed enrollment of 1500 community-dwelling participants aged 70 years and older who are deemed to be at high risk of experiencing mobility disability (40). Eighty researchers from 11 countries are conducting the Phase III randomized controlled trial, which is aimed at preventing mobility disability with a multi-component intervention (MCI) consisting of long-term structured physical activity, personalized nutritional counseling and dietary intervention, and information and communications technology (ICT) intervention. The control group will receive a healthy aging lifestyle education (HALE) program. In designing the SPRINTT trial, researchers proposed a novel operationalization of physical frailty, recognizing sarcopenia as its central biological substrate (41), which should enable identification of a precise target population with unmet medical needs. In addition to evaluating the effectiveness of the multicomponent intervention, the trial also aims to identify and validate diagnostic and prognostic biomarkers for physical frailty related to sarcopenia. The researchers worked closely with the European Medicines Agency (EMA) to develop the study and received EMA’s endorsement of the eligibility criteria and statistical approach and acknowledgment of the rationale driving selection of the target population. The EMA also endorsed the concept of physical frailty related to sarcopenia as a loss of function rather than a set of concomitant disease states, and therefore accepted the incident inability to complete the 400-meter walk test as the primary outcome. EMA has also indicated that if this trial demonstrates benefits in a certain population, they would consider issuing recommendations for industry.
The nutritional intervention component of the SPRINTT will include both an individual nutritional assessment and personalized dietary recommendations, aimed at providing an adequate caloric intake with the appropriate nutrients delivered at the optimal time based on the individual’s age, sex, health status, physical activity level, and comorbidities. The intervention will be delivered for a minimum of 24 months up to a maximum of 36 months. However, while SPRINTT is an important trial, it will be impossible to pull apart the independent effects of exercise and nutritional approaches to combat sarcopenia. Moreover, the nutrition approach is not standardized, which will make it difficult to make definitive statements about the effectiveness of specific nutritional interventions. SPRINTT will be unable to assess the benefits of nutrition on the maintenance of mobility in people with non-physical frailty related to sarcopenia. To understand the effects of nutrition on functional outcomes, trials with different designs will be needed.
Conclusions
The studies conducted so far provide clear evidence that large randomized controlled trials in heterogeneous populations are necessary to quantify in real-world populations the clinical benefit of nutrition on different clinical outcomes, with and without exercise. They also suggest the need to target populations with sarcopenia secondary to undernutrition for nutritional trials. Body mass index can be used for screening but is not a proxy for malnutrition. Subgroups need to be identified at the beginning of a study, i.e., malnourished versus normally nourished, inactive versus active, as well as those with specific conditions such has hip fracture, diabetes, COPD, and obesity (23).
Consensus is also needed on which outcomes are most meaningful and what represents a clinically meaningful change. Regulators have expressed support for including patient-reported outcomes (PROs) in trials because of their focus on clinical meaningfulness. PROs exist to assess the importance of functional improvement to patients, yet no PRO is currently available for malnutrition. A mix of qualitative and quantitative measures that assess both function and independence would be useful. The Task Force could participate in the development of a core battery of measurements to be used in all clinical sarcopenia trials. If trials were conducted in a more standardized way with standard assessments, pooling of data might provide the power necessary to analyze results in subpopulations.
The Task Force split on the question of whether sufficient evidence exists to include nutritional intervention in clinical practice. This will require trials that are convincing enough to be explained easily to patients. While there are substantial data on the benefits of supplemental protein, other nutrients need to be evaluated and studied in more depth. Studies are also needed to demonstrate the benefits of diet versus supplements, nutrition alone versus nutrition in combination with exercise, what kind of exercise is best, and how exercise and supplementation impact total food intake. Despite these limitations of available evidence, there was broad support from the Task Force for improving educational approaches that focus on prevention of sarcopenia through improved nutrition.
Conflicts of interest: JG has consulted for Viking Pharmaceuticals, Pluristem, Boehringer-Ingleheim, and Ammonett Pharma. YR – PRESAGE Study – ANR alia Co-Funding National Research Agency and Lactalis, Consulting fees with Nestlé
Acknowledgements: The authors thank Lisa Bain for assistance in preparing this manuscript.
References
1. Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72(1):45.
2. Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748-59.
3. Cesari M, Fielding R, Benichou O, Bernabei R, Bhasin S, Guralnik JM, et al. Pharmacological Interventions in Frailty and Sarcopenia: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2015;4(3):114-20.
4. Vellas B, Fielding RA, Bens C, Bernabei R, Cawthon PM, Cederholm T, et al. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2018;7(1):2-9.
5. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817-33.
6. Brown JC, Harhay MO, Harhay MN. Physical activity, diet quality, and mortality among sarcopenic older adults. Aging Clin Exp Res. 2017;29(2):257-63.
7. Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, et al. Protein Intake and Mobility Limitation in Community-Dwelling Older Adults: the Health ABC Study. J Am Geriatr Soc. 2017;65(8):1705-11.
8. Landi F, Calvani R, Tosato M, Martone AM, Picca A, Ortolani E, et al. Animal-Derived Protein Consumption Is Associated with Muscle Mass and Strength in Community-Dwellers: Results from the Milan EXPO Survey. J Nutr Health Aging. 2017;21(9):1050-6.
9. Kiesswetter E, Pohlhausen S, Uhlig K, Diekmann R, Lesser S, Heseker H, et al. Malnutrition is related to functional impairment in older adults receiving home care. J Nutr Health Aging. 2013;17(4):345-50.
10. Drey M, Wehr H, Wehr G, Uter W, Lang F, Rupprecht R, et al. The frailty syndrome in general practitioner care: a pilot study. Z Gerontol Geriatr. 2011;44(1):48-54.
11. Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29(1):43-8.
12. Cramer JT, Cruz-Jentoft AJ, Landi F, Hickson M, Zamboni M, Pereira SL, et al. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J Am Med Dir Assoc. 2016;17(11):1044-55.
13. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542-59.
14. Moore DR, Burd NA. Exercise intensity matters for both young and old muscles. J Physiol. 2009;587(3):511-2.
15. Walrand S, Guillet C, Salles J, Cano N, Boirie Y. Physiopathological mechanism of sarcopenia. Clin Geriatr Med. 2011;27(3):365-85.
16. Fulgoni VL, 3rd. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr. 2008;87(5):1554S-7S.
17. Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769-75.
18. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250-8.
19. Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513-20.
20. Timmerman KL, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008;11(1):45-9.
21. Borack MS, Volpi E. Efficacy and Safety of Leucine Supplementation in the Elderly. J Nutr. 2016;146(12):2625S-9S.
22. Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr Metab (Lond). 2008;5:1.
23. Vellas B, Fielding R, Bhasin S, Cerreta F, Goodpaster B, Guralnik JM, et al. Sarcopenia Trials in Specific Diseases: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2016;5(4):194-200.
24. Castaneda C, Gordon PL, Fielding RA, Evans WJ, Crim MC. Marginal protein intake results in reduced plasma IGF-I levels and skeletal muscle fiber atrophy in elderly women. J Nutr Health Aging. 2000;4(2):85-90.
25. Tieland M, Franssen R, Dullemeijer C, van Dronkelaar C, Kyung Kim H, Ispoglou T, et al. The Impact of Dietary Protein or Amino Acid Supplementation on Muscle Mass and Strength in Elderly People: Individual Participant Data and Meta-Analysis of RCT’s. J Nutr Health Aging. 2017;21(9):994-1001.
26. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16(9):740-7.
27. Bo Y, Liu C, Ji Z, Yang R, An Q, Zhang X, et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin Nutr. 2018.
28. Fielding RA, Travison TG, Kirn DR, Koochek A, Reid KF, von Berens A, et al. Effect of Structured Physical Activity and Nutritional Supplementation on Physical Function in Mobility-Limited Older Adults: Results from the VIVE2 Randomized Trial. J Nutr Health Aging. 2017;21(9):936-42.
29. Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, et al. Effect of Protein Intake on Lean Body Mass in Functionally Limited Older Men: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(4):530-41.
30. Englund DA, Kirn DR, Koochek A, Zhu H, Travison TG, Reid KF, et al. Nutritional Supplementation With Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J Gerontol A Biol Sci Med Sci. 2017;73(1):95-101.
31. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772-4.
32. Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29(1):18-23.
33. Kemmler W, von Stengel S, Engelke K, Sieber C, Freiberger E. Prevalence of sarcopenic obesity in Germany using established definitions: Baseline data of the FORMOsA study. Osteoporos Int. 2016;27(1):275-81.
34. Goisser S, Kemmler W, Porzel S, Volkert D, Sieber CC, Bollheimer LC, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons–a narrative review. Clin Interv Aging. 2015;10:1267-82.
35. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18-26.
36. Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103(3):830-40.
37. Ballesteros-Pomar MD, Martinez Llinas D, Goates S, Sanz Barriuso R, Sanz-Paris A. Cost-Effectiveness of a Specialized Oral Nutritional Supplementation for Malnourished Older Adult Patients in Spain. Nutrients. 2018;10(2).
38. Imboden MT, Swartz AM, Finch HW, Harber MP, Kaminsky LA. Reference standards for lean mass measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS One. 2017;12(4):e0176161.
39. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387-96.
40. Landi F, Cesari M, Calvani R, Cherubini A, Di Bari M, Bejuit R, et al. The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. 2017;29(1):89-100.
41. Marzetti E, Calvani R, Landi F, Hoogendijk EO, Fougere B, Vellas B, et al. Innovative Medicines Initiative: The SPRINTT Project. J Frailty Aging. 2015;4(4):207-8.