E. Sachs1, P. St. John2,3, A. Swift1, R. Tate1,3
1. Department of Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Canada
2. Section of Geriatric Medicine, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Canada; 3. Centre on Aging, University of Manitoba, Canada.
Corresponding author: Elizabeth Sachs, Manitoba Follow-up Study, University of Manitoba, P218-770 Bannatyne Avenue, Winnipeg, MB, R3E 0W3, Ph.:204-298-1059, Fax: 204-789-3905, Elizabeth.Sachs@umanitoba.ca
J Frailty Aging 2021;10(1)44-48
Published online March 30, 2020, http://dx.doi.org/10.14283/jfa.2020.14
Abstract
Background: While a multitude of definitions and operationalizations of frailty have been developed, rarely have these considered the perspective of the older adult themselves. This knowledge gap was addressed by examining older adults’ self-rating of frailty. Objectives: To assess the validity of self-rated frailty and to determine whether self-rated frailty relates to mortality. Design: The Manitoba Follow-up Study was initiated in 1948 as a prospective cohort study of 3,983 men. Setting: Community dwelling older adult men. Participants: Survivors of the original cohort (231 men) were sent a quality of life survey in 2015. A response was received from 186 men, including 146 surveys completed by the participant himself and thus were eligible to include (completion rate of 78.4%). Measurements: The quality of life survey is sent out annually to the study participants to ascertain information about mental, physical, and social functioning. In 2015, the Clinical Frailty Scale was adapted and added to the survey as a simple self-rating of frailty. Results: The mean age of the 146 respondents in 2015 was 93.7 years (SD 2.7) Self-ratings of “moderate-severe” frailty, received from 132 men, were associated with worse measures of physical health and functional impairment, thus supporting the significance of self-rated frailty. Adjusted for age, the Hazard Ratio for mortality over the next 3 years was 3.3 (95% CI: 1.5, 7.1) for those who rated themselves as “mildly to severely frail” vs. “very fit or well, with no disease”. Conclusion: The present study has illustrated that self-rated frailty is associated with other measures of health and that self-rated frailty predicts mortality over a three-year period. These findings support the utilization of older adult’s self-ratings of frailty for new avenues of operationalizing frailty.
Key words: Frailty, self-rated, older men, mortality.
Introduction
Frailty has been considered to be a loss of reserve capacity and resistance to stressors (1). This condition has been associated with an increased risk of adverse health outcomes including increased risk of falls, disability, hospitalization, and mortality (1–3). Frailty is a growing public health concern. In 2016, 18% of the United Kingdom’s (UK) population was aged 65 years or older (4). It was estimated that by 2036, this proportion would increase to 24% (4). Similarly, the Canadian older adult population is expected to comprise up to 25% of the population by 2036 (5). The fastest growing segment of this population, the oldest old (80+ years old), are at increased risk for the detrimental effects of frailty (1, 6, 7). Therefore the multifaceted clinical and societal consequences of frailty are expected to increasingly impact the provision and financial implementation of health policy and service provision (8).
Many definitions and models of frailty have been developed. From a medical researcher and clinician perspective, the most popular models are:1) Fried et al.’s (1) frailty phenotype, which considers frailty as a biological syndrome; and 2) Rockwood and Mitnitski’s (9) accumulation of deficits model which views frailty as a state of risk determined by the burden of deficits in multiple domains acquired over time (2, 10). A universal definition of frailty has yet to be agreed upon (11–13), and efforts to reach consensus have had limited success (14).
The study of successful aging experienced similar conceptual and operational challenges (15). In response, Swift and Tate (16) found that lay definitions of successful aging – definitions from the perspective of the older adult – were much more complete than researcher-generated definitions. In light of this parallel research in the area of successful aging, perhaps lay definitions of frailty may be much more comprehensive than researcher-generated conceptualizations.
However, this approach has been minimally explored. Grenier (17) conducted a study exploring lived experiences of frailty of older women. The women interviewed discussed times when they experienced vulnerability and uncertainty, thus “feeling frail” as opposed to elaborations of physical characteristics (17). St. John, McClement, Swift, and Tate (18)explored older men’s definitions of frailty. It was found that 56% of participants did not think that they were frail (18). The participants were also asked to provide their own definition of frailty (18), which did not fully align with clinical definitions (18). Of the responses that did align with a clinical definition, the most popular definition was “frailty as a disability” (18, 19).
Self-rated Frailty
Self-rated health has been well recognized as a valid indicator of health and an important predictor of mortality and well-being (20–22). Analogous to how self-rated health has a strong positive gradient with risk of mortality (20–22), it may be that self-rated frailty (SRF) may also exhibit a relationship with mortality and well-being. This approach has been minimally explored in the literature. With these considerations, we sought to explore the utility of SRF in a Canadian older adult population. The objectives of the present study were to assess the validity of self-rated frailty and to determine whether and how SRF relates to mortality.
Methods
Sample
The Manitoba Follow-up Study (MFUS) is the longest running prospective investigation of cardiovascular disease and aging in Canada. Currently in its 72nd year, this prospective cohort study examines health and well-being in a cohort of Second World War Royal Canadian Air Force aircrew recruits. The cohort was sealed on July 1, 1948 with 3,983 men (23). Further cohort details are available elsewhere (23). The present analysis has used primary data collected from MFUS. In 1996, a quality of life survey was designed and mailed to study participants to ascertain core information about each man’s mental, physical, and social functioning apart from physician diagnosed disease (23). Deemed the Successful Aging Questionnaire (SAQ), the self-administered questionnaire captured several aspects of health, well-being, and functional status (24). The construction of the SAQ drew from several pre-existing sources, most notably the RAND SF-36 (25). Several open-ended qualitative questions assessing successful aging and frailty have since been added to the SAQ (24).
The 2015 SAQ was mailed to 231 members. Of the surveys returned, 23 surveys were received blank, marked “moved” or “deceased.” 148 were filled out by the MFUS member himself without any outside assistance, however 2 additional responses were excluded as the response received was not pertinent (i.e. something other than the SAQ). Therefore, 146 men were included for this analysis at Tinitial. This process was repeated in 2016 and 2017 (Tfinal). A detailed description of the methods of that study are available elsewhere (18).
Measures
Information of interest included limitations with basic activities of daily living (BADL), limitations with instrumental activities of daily living (IADL), mental health (MCS), physical health (PCS), and the study member’s self-rating of frailty (16, 23, 24). Self-rated frailty was measured using a version of the 7-point Clinical Frailty Scale used in Canadian Study of Health and Aging (26, 27), which we modified for self-report. This scale asked participants to “Please rate YOUR frailty on this scale”. Available responses range from 1= very fit to 7= severely frail. This scale is available in Appendix 1.
Data Analysis
Data was analyzed using SAS (version 9.4) in a secure location on the University of Manitoba campus. Hypothesis testing was conducted at the p ≤ 0.05 level of significance. If a SAQ was not returned by the member, the member was excluded from analysis for that year. Responses to the SRF question were coded as ‘missing’ if the SAQ was returned but this question was not answered, or if the given answer was not one of the options available (i.e. a written answer, question crossed out, or “see previous”). Missing answers were excluded from analysis. A questionnaire containing more than one indicated response was assigned the most severe rating.
Following preliminary descriptive analysis, the utility of using a self-rating to measure frailty was investigated by determining how the self-rated Clinical Frailty Scale related to other global measures of health. Specifically, the mean and standard deviation of the measures of health and function assessed within the SAQ (MCS, PCS, IADL, and BADL) were calculated within the categories of self-rated frailty (levels 1-7). These means were then compared with ANOVA. It was expected that if self-rating was a useful measure of frailty, then MFUS members with lower self-rated frailty scores (i.e. less frail) would have better measures of health (higher MCS and PCS scores) and fewer limitations (lower BADL and IADL scores). “Age” was also tested to see if it is significantly related to self-rated frailty. Additionally, the self-rated frailty score from Tinitial and mortality data at Tfinal was used to investigate the relationship between SRF and mortality. A Kaplan-Meier curve illustrated the survival of each grouping of the self-rated frailty scores. Cox proportional hazard modeling illustrated the contributions of self-rated frailty to mortality. Other factors included in the modeling included age, marital status, PCS, and MCS.
Results
The final samples sizes used for analysis were 146 (Tinitial) and 87 (Tfinal). Response rates were 80.5% (Tinitial) and 80.6% (Tfinal). The mean age of the participants was 93.7 (SD 2.7) (Tinitial), and 94.6 (2.7) (Tfinal).
Validity of SRF
The results of the ANOVA that compared the measures of health within categories of the SRF scores are presented by Table 1. After exclusions, the remaining sample sizes were 132 responses at Tinitial. Groups 6 and 7 were combined during analysis, as there were fewer than 5 members reported in group 7. There were statistically significant mean differences for PCS, IADL, and BADL across the six categories of SRF. To determine which group means differ significantly, a post hoc Tukey test was performed. No significant difference in mean PCS was apparent for SRF groups 1 and 2, group 2 was different from group 4, and the mean in each of the first 5 SRF groups differed from the mean PCS of the most frail, group 6 and 7. Mean IADL of the most frail men, group 6 and 7, differed significantly from all other SRF groups. Similarly, the mean BADL of the most frail men in groups 5, 6 and 7 differed significantly from all other SRF groups. These results indicate that the least frail men (groups 1 and 2) reported significantly better physical health than the most frail men (group 6&7). The least frail and most frail men also reported significantly different physical health than men who reported mild frailty (groups 3 and 5). Therefore a gradual gradient of declining physical health with increasing SRF was observed. This gradual change can also be seen within the IADL and BADL variables. Therefore we concluded that increased SRF scores generally correspond with worse health and increased activity limitations as measured by other accepted measures of health (PCS, IADL, BADL).
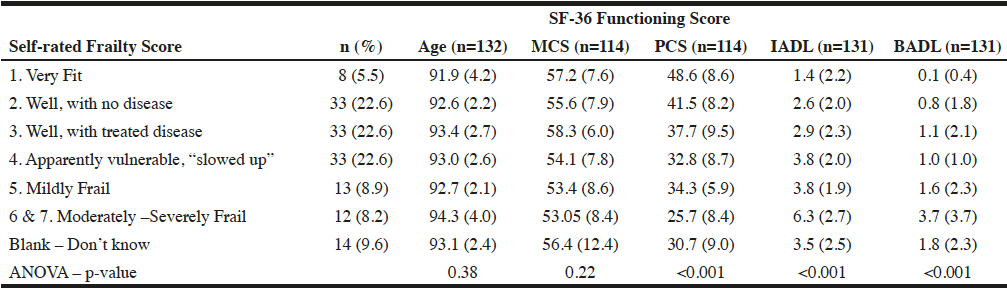
Table 1
Mean and Standard Deviation of Measures of Functional Status (TInitial) Within Categories of Self-rated Frailty Scores
Notes. MCS=Mental Component Score, PCS=Physical Component Score, IADL=Instrumental Activities of Daily Living score, BADL=Basic Activities of Daily Living score. Several health measures use smaller sample sizes because of missing data (i.e. unanswered questions throughout the SAQ); these instances have been indicated as such. MCS/PCS variables were scored so that a lower score indicates worse health. IADL and BADL variables were scored so that a lower score indicated fewer limitations.
Mortality
This study also sought to determine whether and how self-rated frailty relates to mortality. Figure 1 is a Kaplan-Meier curve displaying the survival of each grouping of the self-rated frailty scores (log rank χ2 test: 16.2, 3 df, p<0.001). Cox proportional hazard modeling was used to illustrate the contributions of SRF to mortality. Marital status, MCS score, and PCS score were not significant in multivariable modelling. Table 2 illustrates that the hazard of dying for men who reported a SRF of Mildly-severely frail was 3.3 (95% CI: 1.5, 7.1) times than that of men who reported a SRF of Very fit-well with no disease, when adjusted for age. When men in the Very fit-well with no disease rating were compared to all men in the less fit categories, the age-adjusted hazard ratio was 2.9 (95% CI: 1.6, 5.2). Therefore self-identified frail men have a significantly increased risk of mortality than men who rate themselves as less frail.
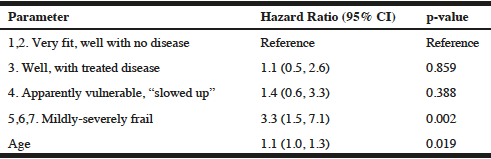
Table 2
Age-adjusted Hazard Ratios (95% CI) for Total Mortality Associated with Categories of Self-rated Frailty at TInitial
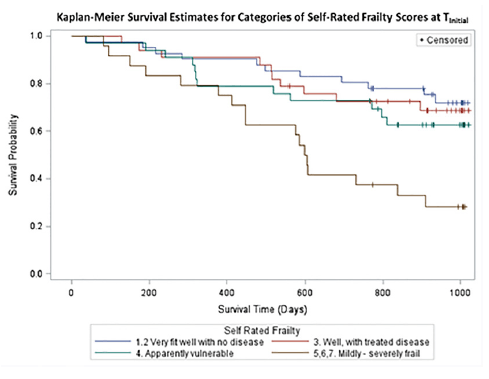
Figure 1
Survival Probability by Self-rated Frailty Group. This figure displays the Kaplan-Meier curve survival of each self-rated frailty grouping. Log rank χ2 test: 16.2, 3 df, p<0.001
Discussion
We explored the utility of SRF in response to conceptual and operation difficulties within the literature. We found that in a cohort of older Canadian men, increased SRF was associated with worse health and increased functional limitations, as measured by other accepted measures of health. We also found that men who self-rated themselves as frail had a significantly increased risk of mortality compared to men who self-rated as less frail. Therefore, in addition to utilizing scales and comprehensive assessments of frailty clinicians and researchers are urged to consider their patient’s self-perceptions of their own experience of frailty.
Strengths
There are several strengths to this study. First, the SAQ used by the present study has been in use at MFUS since 1996, using the same methodology with few deviations since its implementation (15). Furthermore, the men involved with MFUS are familiar with the questionnaire and answering open ended questions. Second, the SAQ is a self-administered questionnaire that has captured several aspects of health, well-being, and functional status (24), and is based on well-established measures such as the SF-36. Third, the data provided by MFUS was unique as studies with participants over the age of 90 are unusual (18, 23).
Limitations
There are several limitations to the present study. First, the sample was made of very old Canadian men (23, 28). Their experiences of frailty may include factors that have a cultural or gendered perspective, impacting the applicability of the present study’s results to older women and to older men from other cultures (18). Secondly these men were born within only a few years of each other, have resided mostly within Canada for most of their lifespan, and have had the common experience of having served in the Royal Canadian Air Force during the Second World War (15, 18). This may limit the generalizability of results to populations outside this demographic.
Implications
As the population ages, an increasing proportion of older adults are expected to be affected by frailty (8). The operational and conceptual definitions in the literature exhibit lack of consensus, limiting the effectiveness of our approach. The present study has provided support that SRF is most closely associated with factors of physical health and functional limitations. Therefore the implications of the present study support that SRF is most closely associated with physical factors or experiences of frailty. In this manner the present study has provided evidence to support operational or conceptual approaches to frailty that consider factors of physical health, such as Fried et al.’s (1) phenotype of frailty or the use of physical components of health in frailty indices (9). Additionally, Table 1 indicates a lack of association between chronological age and severity of SRF score. Although the age range of the sample is narrow, this may show the importance of considering other factors than just age in clinical decision making.
The British Geriatrics Society Fit for Frailty report has acknowledged the importance of identifying the impact frailty has on care provision (29). However, a hesitancy to use the term “frail” when engaging with older adults has been noted (18). This discomfort may be due to fear of offence thereby impacting the physician-patient relationship, the displeasure of delivering bad news, or concern that the patient might internalize a sick role. This study has shown that a self-rating was a useful measure of frailty. Furthermore, the hazard of dying for men who reported a SRF of 5, 6, or 7 at TInitial (group 5,6,7. Mildly-severely frail) was 3.3 (95% CI: 1.5, 7.1) times greater than men who reported a SRF of 1 or 2 (when adjusted for age). As such, the consequences of becoming frail warrant reconsideration on the discussion of SRF with older adults.
Conclusion
In closing, the growing impact of frailty has far reaching implications on the provision and financial implementation of health policy and service provision (8). While several definitions and operationalizations of frailty have been developed, the current researcher-generated definitions of frailty might not fully address the issue. Analogous to the usefulness of self-rated health, using older adult’s self-ratings of frailty may present new avenues of operationalizing frailty. The present study addressed these issues through investigation of the utility of self-rated frailty using data collected from the Manitoba Follow-up Study.
The analyses of the present study showed that increased ratings of SRF scores generally correspond with worse health and increased limitations as measured by other accepted measures of health (PCS, IADL, BADL). It was also found that self-identified frail men have a significantly increased risk of mortality than non-frail men. The implication of these results is that SRF may provide an alternative method that may not be as affected by feasibility concerns during clinical application. Additionally, this project adopted the perspective of the older adult, which was lacking from the current literature.
Funding: This work was funded by the Canadian Institute for Health Research [grant number PJT-152874] and charitable donations from the participants and families of MFUS members. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Acknowledgements: We express our gratitude to the participants of MFUS and their families for their continued involvement in the study.
Conflict of interest: None declared by the Authors.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol Med Sci. 2001;56A(3):M146–56.
2. Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013;14(6):392–7.
3. Chen X, Mao G, Leng SX. Frailty syndrome: An overview. Clin Interv Aging. 2014;9:433–41.
4. Office for National Statistics. Overview of the UK Population: July 2017. Office for National Statistics. 2017.
5. Statistics Canada. An aging population [Internet]. 2016 [cited 2017 May 31]. Available from: http://www.statcan.gc.ca/pub/11-402-x/2010000/chap/pop/pop02-eng.htm
6. Choi J, Ahn A, Kim S. Global Prevalence of Physical Frailty by Fried’s Criteria in Community-Dwelling Elderly With National Population-Based Surveys. J Am Med Dir Assoc. 2015;16(7):548–50.
7. Canadian Frailty Network. What is Frailty? [Internet]. [cited 2019 Jul 2]. Available from: https://www.cfn-nce.ca/frailty-matters/what-is-frailty/
8. Buckinx F, Rolland Y, Reginster J-Y, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Heal. 2015;73(1):19.
9. Rockwood K, Mitnitski AB. Frailty Defined by Deficit Accumulation and Geriatric Medicine Defined by Frailty. Clin Geriatr Med. 2011;27(1):17–26.
10. Theou O, Brothers TD, Mitnitski AB, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–51.
11. Sternberg SA, Schwartz AW, Karunananthan S, Bergman H, Clarfield AM. The identification of frailty: A systematic literature review. J Am Geriatr Soc. 2011;59(11):2129–38.
12. Cesari M, Gambassi G, Van Kan GA, Vellas B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing. 2014;43(1):10–2.
13. Gobbens RJJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. Determinants of Frailty. J Am Med Dir Assoc. 2010 Jun;11(5):356–64.
14. Gobbens RJJ, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. In Search of an Integral Conceptual Definition of Frailty: Opinions of Experts. J Am Med Dir Assoc. 2010 Jun;11(5):338–43.
15. Tate RB, Swift AU, Bayomi DJ. Older Men’s Lay Definitions of Successful Aging Over Time: The Manitoba Follow-Up Study. Int J Aging Hum Dev. 2013;76(4):297–322.
16. Swift AU, Tate RB. A bidirectional approach to lay definitions vs. theories of successful aging: The Manitoba Follow-up Study. Heal Aging Res. 2015;4(38):1–13.
17. Grenier AM. Constructions of frailty in the English language, care practice and the lived experience. Ageing Soc. 2007;27(03):425–45.
18. St. John PD, McClement SS, Swift AU, Tate RB. Older Men’s Definitions of Frailty – The Manitoba Follow-up Study. Can J Aging / La Rev Can du Vieil. 2019;38(1):13–20.
19. Cosin L. Organizing a geriatric department. Br Med J. 1947;2(4538):1044–6.
20. Mossey JM, Shapiro E. Self-rated health: A predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800–8.
21. Manor O, Matthews S, Power C. Self-rated health and limiting longstanding illness: inter-relationships with morbidity in early adulthood. Int J Epidemiol. 2001;30(3):600–7.
22. Lucicesare A, Hubbard RE, Searle SD, Rockwood K. An index of self-rated health deficits in relation to frailty and adverse outcomes in older adults. Aging Clin Exp Res. 2010;22(3):255–60.
23. Tate RB, Cuddy TE, Mathewson FAL. Cohort Profile: The Manitoba Follow-up Study (MFUS). Int J Epidemiol. 2015;44(5):1528–36.
24. Tate RB, Lah L, Cuddy TE. Definition of Successful Aging by Elderly Canadian Males: The Manitoba Follow-Up Study. Gerontologist. 2003;43(5):735–44.
25. Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I . Conceptual Framework and Item Selection. Med Care. 1992;30(6):473–83.
26. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–95.
27. Cheung A, Haas B, Ringer TJ, McFarlan A, Wong CL. Canadian Study of Health and Aging Clinical Frailty Scale: Does It Predict Adverse Outcomes among Geriatric Trauma Patients? J Am Coll Surg. 2017;225(5):658–665.e3.
28. Tate RB, Loewen BL, Bayomi DJ, Payne BJ. The consistency of definitions of successful aging provided by older men: The Manitoba follow-up study. Can J Aging. 2009;28(4):315–22.
29. British Geriatrics Society. Fit for Frailty Part 1: Consensus best practice guidance for the care of older people living in community and outpatient settings. 2017.
