O. Addison1,2, A.S. Ryan1,3, J. Blumenthal1, S.J. Prior1,4
1. Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore, Geriatric Research, Education and Clinical Center (GRECC), Baltimore, MD, USA; 2. Department of Physical Therapy and Rehabilitation Science, University of Maryland School of Medicine, Baltimore, MD, USA; 3. Department of Medicine, Division of Gerontology and Geriatric Medicine, University of Maryland School of Medicine, Baltimore, MD, USA; 4. Department of Kinesiology, University of Maryland School of Public Health, College Park, MD, USA.Corresponding author: Odessa Addison, 10 N. Greene Street, Baltimore, MD, 21201, Phone- (410)605-7000 ext 55393, Fax: (410)605-7913, Email – oaddison@som.umaryland.edu
J Frailty Aging 2020;9(3)134-138
Published online April 24, 2020, http://dx.doi.org/10.14283/jfa.2020.20
Abstract
Background: High levels of intramuscular adipose tissue and low levels of capillarization are both predicative of low muscle and mobility function in older adults, however little is known about their relationship. Objectives: The purpose of this study was to examine the relationship of intramuscular adipose tissue and capillarization in older adults. Setting: An outpatient medical center. Participants: Forty-seven sedentary adults (age 59.9 ± 1.0 years, BMI 32.0 ± 0.7 kg/m2, VO2max 22.4 ± 0.7 ml/kg/min); Measurements: All participants underwent CT scans to determine intramuscular adipose tissue and muscle biopsies to determine capillarization in the mid-thigh. A step-wise hierarchical linear regression analysis was used to examine the contributions of age, sex, race, body mass index, 2-hour postprandial glucose, VO2max, and muscle capillarization, to the variability in intramuscular adipose tissue. Results: The predictors as a group accounted for 38.1% of the variance in intramuscular adipose tissue, with body mass index and capillarization each significantly contributing to the final model (P<0.001). The part correlation of body mass index with intramuscular adipose tissue was r = 0.47, and the part correlation of capillarization with intramuscular adipose tissue was r = 0.39, indicating that body mass index and capillarization explained 22.1%, and 15.2% of the variance in intramuscular adipose tissue. Conclusions: While increased muscle capillarization is typically thought of as a positive development, in some clinical conditions, such as tendinopathies, an increase in capillarization is part of the pathological process related to expansion of the extracellular matrix and fibrosis. This may also be an explanation for the surprising finding that high capillarization is related to high levels of intramuscular adipose tissue. Future studies are necessary to determine the relationship of changes in both capillarization and intramuscular adipose tissue after interventions, such as exercise.
Key words: Myosteatosis, muscle, vascular.
Introduction
Aging is associated with numerous muscular changes including a loss of muscle strength and lean mass, and an increase in muscle intramuscular fat (IMAT), also known as myosteatosis (1). Changes in muscles composition are an important factor related to metabolism, strength, and physical function in older adults (1). Older adults with high levels of IMAT often have insulin resistance, decreased muscle quality and physical function, and an increased risk for future mobility limitations (1, 2) . While the cause of increased IMAT is currently unknown, comorbid conditions such as diabetes and obesity, as well as inactivity and disuse all appear to contribute (3). Likewise, lifestyle interventions such as weight loss, exercise, and physical activity interventions appear to ameliorate the accumulation, or even result in reductions in IMAT (4) .
Capillarization in skeletal muscle is also an important factor associated with metabolism and muscle function in older adults (5). Low skeletal muscle capillarization is associated with sarcopenia (6), increased insulin resistance (7), and low physical function (8). Decreases in skeletal muscle capillarization also occur with aging (9), sedentary behavior (10), and in co-morbid conditions such as diabetes (11). Similarly, higher aerobic capacity is associated with increased capillarization in skeletal muscle, and exercise may likewise increase capillarization in previously sedentary older adults (12). Due to the close proximity of IMAT with the skeletal muscle, and the secretion of pro- and anti-angiogentic factors from adipose tissue (13), IMAT may exert an influence on the capillarization of a muscle.
It is reasonable to suspect that high levels of IMAT may be related to decreased capillarization in skeletal muscle; however, the relationship between the two variables has not yet been examined. Understanding this relationship is important for the effective development of treatment to both decrease IMAT and increase capillarization in the muscle of older adults. Therefore, the purpose of this paper is to examine the relationship of IMAT and capillarization in the thigh muscle of older adults. We hypothesize that low levels of capillarization would be associated with high levels of IMAT.
Methods
This study was conducted as a secondary data analysis from a previously published study (6). In brief, participants age 45-80 who participated in studies examining the metabolic responses to exercise or exercise and weight loss, and had complete baseline data for muscle composition, exercise capacity, and capillarization were included, resulting in 47 participants with an age range of 50-77 years for this study. Only baseline data were used in this cross-sectional analysis. Individuals were included in this study if they were non-smokers who were weight stable (self-reported weight change of <2.0 kg in the last year), sedentary (<20 minutes of aerobic exercise two times per week), and free from diabetes (confirmed with an oral glucose tolerance test), stroke, coronary artery disease, heart failure, peripheral arterial disease, and liver, kidney or lung disease. Health status was confirmed in a physical examination performed by a physician or nurse practitioner that included a medical history, fasting blood chemistry, a graded maximal exercise test, and a two-hour fasting oral glucose tolerance test. All participants signed written informed consent approved by the University of Maryland Baltimore Institutional Review Board.
Body Mass Index and Muscle Composition
Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). Cross-sectional area (cm2) of both high and low-density lean tissue was determined utilizing computed tomography (CT). The methods have previously been reported in detail (2). Briefly, participants underwent a mid-thigh CT scan (Siemens Somatom Sensation 64 Scanner). High (HDLT) and low-density lean tissue (LDLT) cross-sectional area of the thigh were determined using Medical Image Processing, Analysis and Visualization (MIPAV, v 7.0, NIH) software. (14) CT data for each muscle were expressed as a cross-sectional area of tissue (cm2) using Hounsfield units 30-100 for HDLT and 0-29 for LDLT. LDLT was normalized to thigh size by calculating a percentage of LDLT (% LDLT) relative to the sum of LDLT and HDLT (LDLT/(LDLT+HDLT). As done previously, %LDLT was used as a measure of IMAT (14).
Exercise Capacity
During a maximal graded treadmill exercise test, VO2max was measured by indirect calorimetry (Quark, Cosmed USA, Chicago, IL) as previously described (12). In brief, participants walked at a constant speed during the test with a starting grade of 0%, the grade was increased every two minutes until a maximal effort was achieved. VO2max was verified using standard physiological criteria (i.e., respiratory exchange ratio >1.10 or a plateau in VO2 with increased workload).
Capillarization
Skeletal muscle capillarization was determined using percutaneous muscle biopsies from the vastus lateralis. Using a Bergstrom needle (Stille, Solna, Sweden), muscle samples were obtained from 12-13 cm above the patella on the right thigh (12). Muscle samples were embedded and rapidly frozen in optimal cutting temperature-tragacanth gum mixture, then stored at -80° C for histochemical analyses. Samples were sectioned to a thickness of 14 µm on a cryostat, and capillaries were identified using a modified double stain technique in our laboratory as described previously (12). Three measures of capillarization were obtained: 1) capillary density (CD: the number of capillaries per mm2 of muscle cross-sectional area), 2) the capillary to fiber ratio (C:F: the number of whole capillaries equivalents in contact with each muscle fiber) and 3) the capillary-to-fiber perimeter exchange index (CFPE: the number of capillaries per millimeter of muscle fiber perimeter). CFPE was chosen as the primary measurement for analyses as it is thought to best represent the potential for blood-tissue exchange (15).
Statistical Analysis
All statistical analyses were performed using SPSS Statistics v. 22 (IBM, Armonk, NY). Data were ensured to meet the assumptions of a normal distribution prior to all analysis. Descriptive statistics were performed for demographic variables and dependent measures and are presented as mean +/- SEM. Pearson product-moment correlation analyses were used to test for bivariate correlations between %LDLT, muscle capillarization, age, BMI, 2-hr postprandial glucose (G120), and exercise capacity. To assess the relationship of capillarization and IMAT, individuals were divided into tertiles of capillarization and the 1st and 3rd tertial were compared using independent samples t-tests. The alpha level for analyses was set at <0.05.
To further assess the relative contributions of demographic variables (age, sex, race, BMI, G120), muscle capillarization, and VO2max to the variability in %LDLT were examined using a step-wise hierarchical linear regression model. In the model, %LDLT was the dependent variable and age, sex, race, BMI, G120, VO2max, and muscle capillarization, as represented by CFPE, were considered for entry in a stepwise manner. A second step-wise hierarchical linear regression was used to examine the contributions of demographics, VO2max, and %LDLT to variability in muscle capillarization. In this second model, muscle capillarization (CFPE) was the dependent variable and demographics, VO2max, and %LDLT were considered for entry in a stepwise manner. The criterion for entry to both models was a significance level of P<0.10. For each variable entered in the final model, the part-correlation was examined to determine the unique amount of variance in the outcome (%LDLT or muscle capillarization) that was accounted for by the variable.
Results
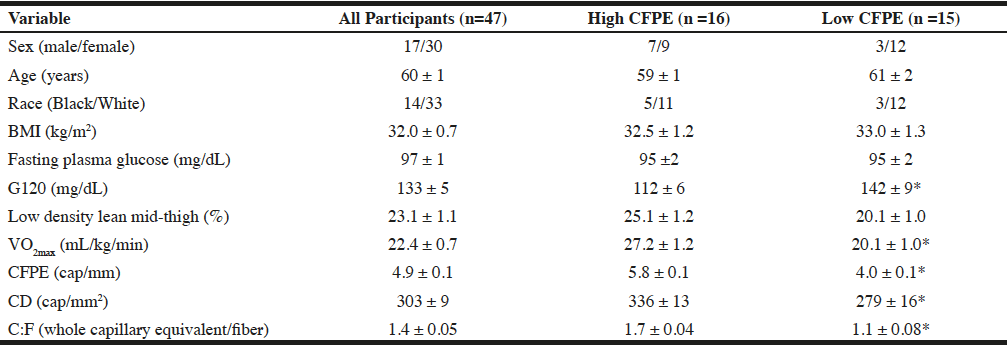
Participants were all middle-aged to older women and men with BMI ranging from 24.2-46.1 kg/m2 and low physical fitness (VO2max range 8.9-33.5 ml/kg/min; Table 1). The bivariate correlations of %LDLT with all other variables revealed moderately strong (r = 0.40-0.48) and significant (p<0.004) correlations with capillarization (Figure 1) and BMI (Table 2). After dividing the groups into tertiles by capillarization there was no significant difference between those in the high and low capillarization groups for age, race, or BMI (table 1). There was however a tendency (p=0.06) for a difference in the %LDLT and a significant difference (P<0.05) in VO2max, and G120 with the higher capillarization group demonstrating higher levels of %LDLT and VO2max and lower G120 levels.
Notes: Data are means ± SEM with the exceptions of sex and race. High CFPE refers to those in the top tertial of CFPE while low CFPE are those in the bottom tertial. BMI: Body mass index; 120-minute postprandial glucose; CFPE: capillary fiber perimeter exchange index; CD: capillary density; C:F: Capillary to fiber ratio. * Significant difference between the high and low CFPE groups (P<0.05)
Note: Bivariate Person correlation coefficients are presented to show the relationships among percentage low density lean and CFPE and other variables. BMI: Body mass index; CFPE: Capillary fiber perimeter exchange index; G120: 120 minute postprandial glucose; %LDLT: percentage of low density lean tissue a representation of intramuscular adipose tissue. *P<0.05
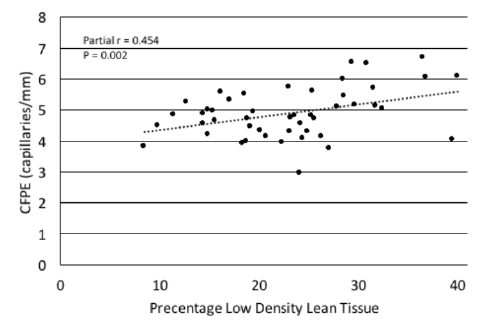
Figure 1
Scatterplot depicting the relationship of the percentage of low density lean tissue with capillary-to-fiber perimeter exchange index (CFPE) in sedentary older adults. In both bivariate correlation and regression analyses %LDLT was directly associated with CFPE
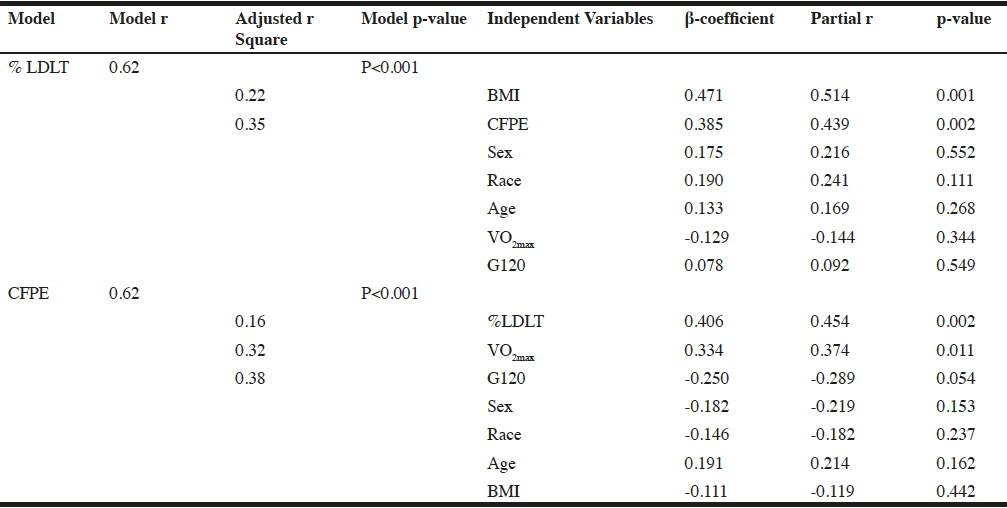
The multiple regression analysis revealed that the predictors as a group accounted for 38.1% of the variance in %LDLT, with BMI (P<0.001) and capillarization (P=0.002), each significantly contributing to the final model (P<0.001). The part correlation of BMI with %LDLT was r = 0.47, and the part correlation of CFPE with %LDLT r = 0.39, indicating that BMI and capillarization explained 22.1%, 15.2% of the variance in %LDLT respectively, with all other variables in the model held constant (Table 3).
For the second regression analysis, the predictors as a group accounted for 38.0% of the variance in CFPE, with %LDLT (P<0.002), VO2max (P=0.01), and G120 (P=0.05) each significantly contributing to the final model (P<0.001). In this model, the part correlation of %LDLT with CFPE was r = 0.40, of VO2max with CFPE was r = 0.32, and of G120 with CFPE was r = -0.24 indicating that %LDLT, VO2max, and G120 explained 16.0%, 10.1%, and 5.7% of the variance in capillarization, with all other variables in the model held constant (Table 3).
Note: Regression analysis presented to show the relationships of low density lean tissue, a representation of intramuscular adipose tissue, capillarization (defined as CFPE) and other variables. Partial correlation coefficients are presented to show the relationships among low density lean tissue, capillarization and the other variables in the regression analysis. %LDL: percentage of low density lean as a representation of intramuscular adipose tissue; BMI: body mass index; 120-minute postprandial glucose; CFPE: capillary fiber perimeter exchange index
Discussion
Contrary to our original hypothesis, we found that increased amounts of LDLT were related to increased levels of capillarization in the thigh. To our knowledge, this is the first time this relationship has been reported. Our findings that the most significant predictors of %LDLT in the thigh were BMI and capillarization, and that the most significant predictor of capillarization in the thigh was %LDLT are surprising. Previous work has found high levels of IMAT in individuals with compromised microvasculature such as in diabetes, aging, and sedentary behavior (1, 3). Conversely, interventions such as aerobic exercise and weight loss may increase capillarization and decrease IMAT (4, 12). Our paradoxical finding of increased IMAT being related to increased capillarization is surprising in light of this previous literature.
Adipose tissue is known to release a host of proteins that act in endocrine, paracrine, and autocrine signaling including increased inflammatory and angiogenic factors (13). The intimate relationship between IMAT and muscle cells indicates that IMAT may have a unique interactions with muscle (16). Previous work has demonstrated that increased levels of IMAT may promote an increase in the local proinflammatory environment (17), modify the extracellular matrix (16), and ultimately result in muscle fibrosis (18). It is possible that this combination of changes also results in an increase in capillarization within the muscle. Increased intramyocellular lipid levels (one component of IMAT) are found both in athletes (who have high levels of capillarization) and in sedentary obese adults when compared to lean sedentary individuals (19). However, given the low VO2max levels of individuals in this study, they would not be considered athletes and this is an unlikely explanation for our findings.
While increasing capillarization in muscle is typically thought of as a positive development, in some musculoskeletal conditions, such as tendinopathies, an increase in angiogenesis is part of the pathological process. For example, in patellar and Achilles tendinopathies an increase in the microvascular density occurs (20). This increase is related to the expansion of the extracellular matrix and ultimately the fibrosis of the tendon. It is possible that with increased levels of IMAT, the increased capillarization of the muscle is also related to the increased local inflammatory environment and changes in the extracellular matrix. However, as this is a cross-sectional study, this is only speculative and we do not know the nature of the relationship of timing between increased IMAT and capillarization.
As we eliminated any individuals with diabetes from our sample, in an effort to control for the numerous effects of diabetes on both IMAT and capillarization, more studies are necessary to examine the relationships of capillarization and IMAT in those with diabetes. The older adults in this study were also all sedentary individuals and it is possible that if we included active individuals in the study we would find something similar to the athletes paradox.
In conclusion, contrary to our initial hypothesis that high levels of IMAT would be related to low levels of capillarization, we found that high levels of IMAT were related to high levels of capillarization. Given the cross-sectional nature of this study, future studies are necessary to determine the relationship of changes in both capillarization and IMAT with interventions, such as exercise.
Funding: This research was supported by the University of Maryland Claude D. Pepper Center (P30-AG-12583), the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC), the National Institutes of Health (grant numbers R01-AG019319 and R01-AG020116) plus the Department of Veterans Affairs. OA was supported by a Veterans Affairs Career Development Award (IK2RX001788), A.S.R. was supported by a Veterans Affairs Senior Research Career Scientist Award, and S.J.P was supported by a Paul B. Beeson Patient-Oriented Research Career Development Award in Aging (National Institutes of Health K23-AG040775 and AFAR). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; in the preparation of the manuscript, or in the review or approval of the manuscript.
Conflicts of Interest: All the authors (OA, ASR, JB, SJP) declare no conflicts of interest.
References
1. Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. doi:10.1155/2014/309570.
2. Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999 Feb;23(2):126-32.
3. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000 Apr;71(4):885-92. eng. Epub 2000/03/25.
4. Prior SJ, Joseph LJ, Brandauer J, Katzel LI, Hagberg JM, Ryan AS. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007 Mar;92(3):880-6. doi:10.1210/jc.2006-2113.
5. Landers-Ramos RQ, Prior SJ. The Microvasculature and Skeletal Muscle Health in Aging. Exerc Sport Sci Rev. 2018 Jul;46(3):172-179. doi:10.1249/JES.0000000000000151.
6. Prior SJ, Ryan AS, Blumenthal JB, Watson JM, Katzel LI, Goldberg AP. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J Gerontol A Biol Sci Med Sci. 2016 Aug;71(8):1096-101. doi:10.1093/gerona/glw017.
7. Prior SJ, McKenzie MJ, Joseph LJ, Ivey FM, Macko RF, Hafer-Macko CE, Ryan AS. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation. 2009 Apr;16(3):203-12. eng. Epub 2009/02/20. doi:10.1080/10739680802502423.
8. Nicklas BJ, Leng I, Delbono O, Kitzman DW, Marsh AP, Hundley WG, Lyles MF, O’Rourke KS, Annex BH, Kraus WE. Relationship of physical function to vastus lateralis capillary density and metabolic enzyme activity in elderly men and women. Aging Clin Exp Res. 2008 Aug;20(4):302-9.
9. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). 2000 Apr;88(4):1321-6. doi:10.1152/jappl.2000.88.4.1321.
10. Vigelso A, Gram M, Wiuff C, Andersen JL, Helge JW, Dela F. Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J Rehabil Med. 2015 Jun;47(6):552-60. doi:10.2340/16501977-1961.
11. Solomon TP, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab. 2011 May;96(5):1377-84. doi:10.1210/jc.2010-2069.
12. Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased Skeletal Muscle Capillarization After Aerobic Exercise Training and Weight Loss Improves Insulin Sensitivity in Adults With IGT. Diabetes Care. 2014 May;37(5):1469-75. doi:10.2337/dc13-2358.
13. Tahergorabi Z, Khazaei M. The relationship between inflammatory markers, angiogenesis, and obesity. ARYA Atheroscler. 2013 Jun;9(4):247-53. Epub 2013/08/24.
14. Addison O, Inacio M, Bair WN, Beamer BA, Ryan AS, Rogers MW. Role of Hip Abductor Muscle Composition and Torque in Protective Stepping for Lateral Balance Recovery in Older Adults. Arch Phys Med Rehabil. 2017 Jun;98(6):1223-1228. Epub 2016/11/15. doi:10.1016/j.apmr.2016.10.009.
15. Hepple RT. A new measurement of tissue capillarity: the capillary-to-fibre perimeter exchange index. Can J Appl Physiol. 1997 Feb;22(1):11-22. Epub 1997/02/01.
16. Sachs S, Zarini S, Kahn DE, Harrison KA, Perreault L, Phang T, Newsom SA, Strauss A, Kerege A, Schoen JA, Bessesen DH, Schwarzmayr T, Graf E, Lutter D, Krumsiek J, Hofmann SM, Bergman BC. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am J Physiol Endocrinol Metab. 2019 May 1;316(5):E866-E879. Epub 2019/01/09. doi:10.1152/ajpendo.00243.2018.
17. Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014 May;18(5):532-8. Epub 2014/06/03. doi:10.1007/s12603-014-0019-1.
18. McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan. 2014;3(1):9. Epub 2014/12/19. doi:10.1186/2046-2395-3-9.
19. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001 Dec;86(12):5755-61. Epub 2001/12/12. doi:10.1210/jcem.86.12.8075.
20. Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008 Jul;466(7):1528-38. Epub 2008/05/15. doi:10.1007/s11999-008-0286-4.