S. Sourdet1, G. Soriano1,2, J. Delrieu1,2, Z. Steinmeyer1, S. Guyonnet1,2, L. Saint-Aubert3,4, P. Payoux3,4, P.J. Ousset1,2, A. Ghisolfi1, B. Chicoulaa1,5, S. Dardenne1, T. Gemar1, M. Baziard1, F. Guerville6, S. Andrieu1,2, B. Vellas1,2
1. Gérontopôle, Department of Internal Medicine and Geriatrics, Toulouse University Hospital, La Cité de la Santé, Hôpital La Grave, Place Lange, TSA 60033, 31059 TOULOUSE Cedex 9, France; 2. UMR Inserm Unit 1027, University of Toulouse III, Toulouse, France; 3. Department of Nuclear Medicine, Imaging Center, Toulouse University Hospital, Toulouse, France; 4. Toulouse NeuroImaging Center, Inserm UMR1214, Toulouse University, UPS, Toulouse, France; 5. University Department of General Medicine, Paul Sabatier University, Toulouse; 6. Gérontopôle of Toulouse, Institute of Aging, Toulouse University Hospital, Toulouse, France.
Corresponding author: Sandrine Sourdet, MD – Hôpital de jour d’évaluation des fragilités, Service de Médecine Interne et Gérontologie Clinique, La Cité de la Santé, Hôpital La Grave, Place Lange, TSA 60033, 31059 Toulouse cedex 9, France, Phone: (33) 5 61 77 79 29, Fax: (33) 5 61 77 79 27, E-mail: sourdet.s@chu-toulouse.fr
J Frailty Aging 2020;in press
Published online October 13, 2020, http://dx.doi.org/10.14283/jfa.2020.57
Abstract
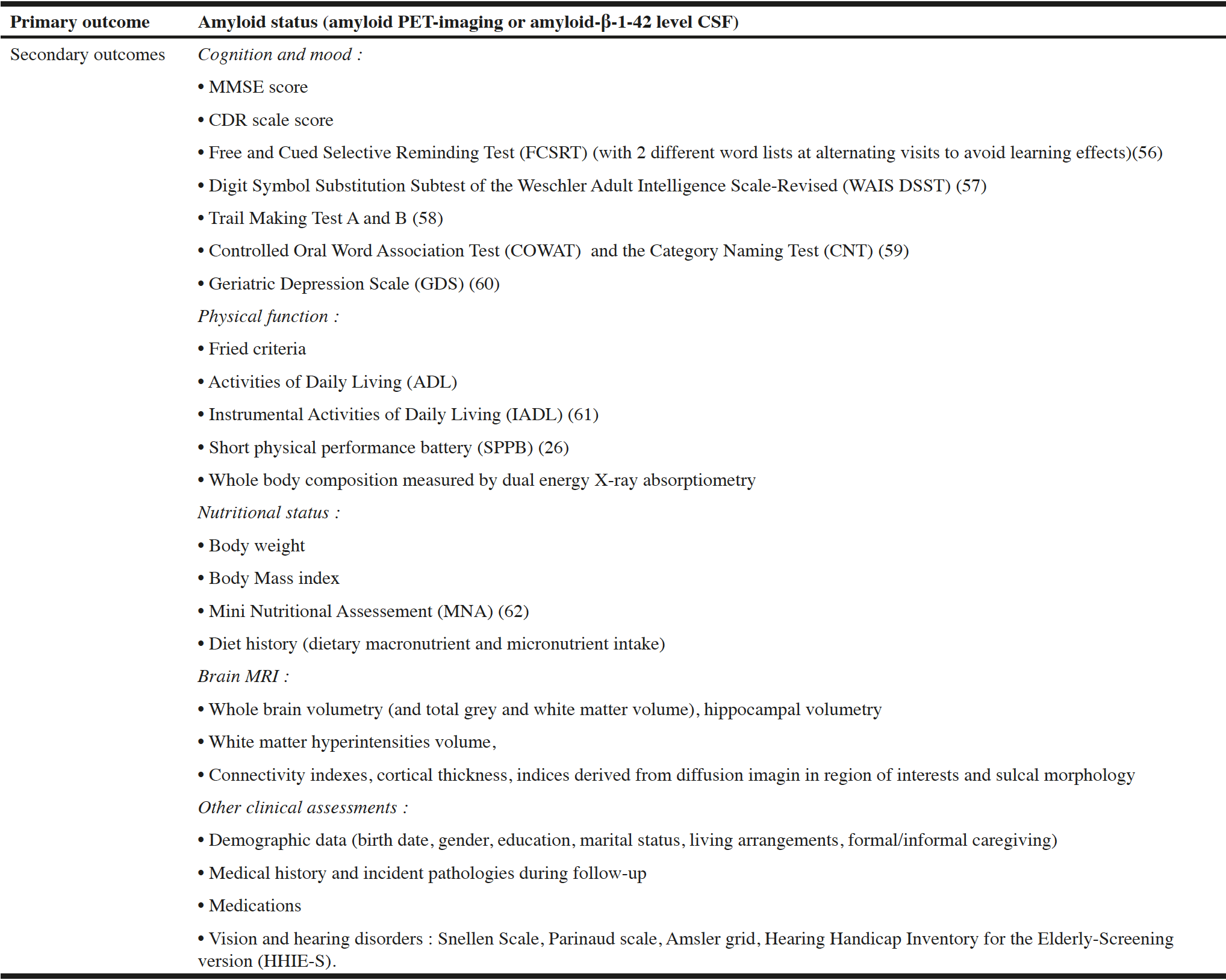
Background: Frailty and cognitive impairment are common manifestations of the ageing process and are closely related. But the mechanisms linking aging, physical frailty, and cognitive disorders, are complex and remain unclear. Objectives: We aim to explore the role of cerebral amyloid pathology, but also a range of nutritional, physical, biological or brain-aging marker in the development of cognitive frailty. Method: COGFRAIL study is a monocentric prospective study of frail older patients with an objective cognitive impairment (Clinical Dementia Rating Scale global score at 0.5 or 1). Three-hundred-and-twenty-one patients are followed up every 6 months, for 2 years. Clinical assessment at baseline and during follow-up included frailty, physical, mood, sensory, nutritional, and cognitive assessment (with a set of neuropsychological tests). Cerebral amyloid pathology is measured by amyloid Positron Emission Tomography (PET) or amyloid-β-1-42 level in cerebrospinal fluid. Brain magnetic resonance imaging, measurement of body composition using Dual X Ray Absorptiometry and blood sampling are performed. The main outcome of the study is to assess the prevalence of positive cerebral amyloid status according to amyloid PET or amyloid-β-1-42 level CSF. Secondary outcomes included biological, nutritional, MRI imaging, cognitive, clinical, physical and body composition markers to better understand the mechanisms of cognitive frailty. Perspective: COGFRAIL study will give the opportunity to better understand the link between Gerosciences, frailty, cognitive impairment, and Alzheimer’s disease, and to better characterize the physical and cognitive trajectories of frail older adults according to their amyloid status. Understanding the relationship between physical frailty and cognitive impairment is a prerequisite for the development of new interventions that could prevent and treat both conditions.
Key words: Cognitive decline, frailty, older adults, Alzheimer’s disease, geroscience, amyloid, neuroimaging.
Introduction
With population ageing, frailty has received growing interest from scientific and clinical communities, as well as by the public health authorities (1). Frailty is best defined as an acceleration of the aging process, a condition of homeostatic degradation across physiological systems leaving the individual at high risk of negative health-related outcomes, including disability, hospitalization and mortality. More recently, a number of cross-sectional and longitudinal studies have suggested that physical frailty was associated with late-life cognitive disorders, including mild cognitive impairment (MCI), non Alzheimer’s disease (AD) dementia, and AD (2, 3). Several mechanisms, known for playing a role in both cognitive impairment and frailty have been proposed to explain this link (4). Vascular risk factors, for example, are known to increase the risk of stroke, cerebrovascular lesions, but also sarcopenia, which are involved in the development of frailty on one hand and the development of vascular dementia but also AD on the other hand (5). Several nutritional factors, such as undernutrition, weight loss, reduced caloric or specific nutrients intake, are also associated with a higher risk of both frailty and cognitive impairment (6–8). Genetic and environmental stressor, depression, hormonal dysfunctions may also play a role (4, 8). Another hypothesis is the existence of a direct link between brain pathology and frailty. Several neuropathological studies, using brain autopsies from the Religious Orders Study and the Rush Memory and Aging Project, have explored the association between physical frailty, cognitive decline and the presence of postmortem neuropathologies (9–11). They reported an association between progressive frailty before death and common brain pathologies including AD pathology, Lewy Body pathology, macroinfarcts and nigral neuronal loss, in older adults with but also without dementia. Focusing on AD biomarkers, PET amyloid imaging studies have also suggested an association between regional brain β-amyloid deposition and frailty parameters (12–14).
Despite mounting evidence that cognitive impairment and frailty are closely connected, and that AD pathology could play a role in their co-expression (15), there are still insufficient data exploring this pathological pathway and their potential confounders, especially in vivo. Moreover, longitudinal data are still lacking to understand the evolution of cognitive impairment in the frail and pre-frail population. The COGFRAIL study is a large clinic-based cohort of frail older adults with cognitive impairment. Investigations include regular clinical, cognitive, physical, nutritional, biological assessment and neuroimaging (amyloid PET and Magnetic Resonance Imaging, MRI). In this study, we seek to better understand the proportion of cognitive decline, which can be explained by an AD pathology in frail older adults. It will also allow us to study the role of a range of aging biomarkers with a geroscience approach.
Methods and analysis
Aim and objectives
The main objective of the study is to examine the prevalence of subjects with a positive cerebral amyloid status according to amyloid PET or amyloid-β-1-42 level cerebrospinal fluid (CSF), among frail and pre-frail individuals presenting an objective cognitive impairment. The key secondary objectives are to examine changes in cognitive function, physical function, frailty status and body composition during follow-up. We also aim to explore the relationship between these parameters (at baseline and their change during follow-up) and 1/MRI markers, 2/food intake, and 3/nutritional and aging biomarkers (including APOE genotype). Other secondary objectives are to explore the association between cognitive decline during follow-up and changes in physical function, sensory impairments, frailty and body composition, These objectives will be explored in the total population, and also according to the amyloid status. A last objective is to estimate costs related to the management of frail and pre-frail older patients presenting an objective cognitive impairment.
Study design
COGFRAIL is a monocentric observational prospective study. The participants are followed up every 6 months, during 2 years after enrolment.
Study population and eligibility criteria
Three hundred twenty-one participants were recruited for this study, between January 2017 and February 2020. Participants were invited to participate during a routine frailty or cognitive assessment, at the Gerontopôle, University of Toulouse Hospital Center, at the Frailty Clinic, Memory Clinic, or during community-care assessment.
The frailty clinic is a geriatric day hospital unit, dedicated to the assessment and follow-up of frail older adults. Patients were referred after having been identified as frail by their general practitioner using the Gerontopôle Frailty Screening Tool (16). Its functioning is well described elsewhere (17). Most of the participants were recruited at the frailty clinic, as the study is designed following the standardized clinical assessment and follow-up implemented in this unit. The enrollment was also extended to community care, through frailty promotion program developed locally (18) and during usual cognitive assessment at the memory clinic.
Inclusion criteria are:
• Having an objective memory impairment defined by a Clinical Dementia Rating (CDR) global score at 0.5 or 1 (19)
• Age ≥ 70 years
• Having at least 1 frailty criteria according to Fried’s criteria (20)
• Having an informant accompanying or available by phone
• Being affiliated to a healthcare security system.
Exclusion criteria are:
• Presence of any pathology or severe clinical or psychological condition that, based on medical judgment, might interfere with study results or may expose the participants to additional risks
• Dependency (Activities of Daily Living (ADL) <4) (21)
• A major deterioration in global cognitive function (Mini Mental State Examination (MMSE<20) at inclusion (22)
• Adults legally protected (under judicial protection, guardianship or supervision).
Study outcomes and data collection
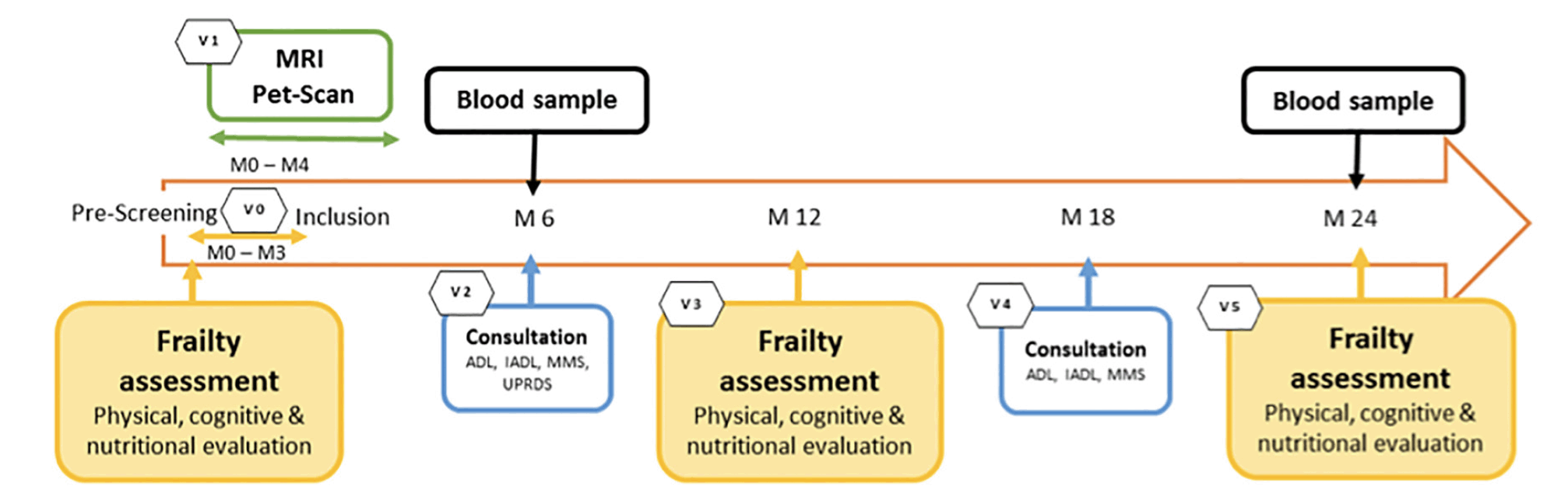
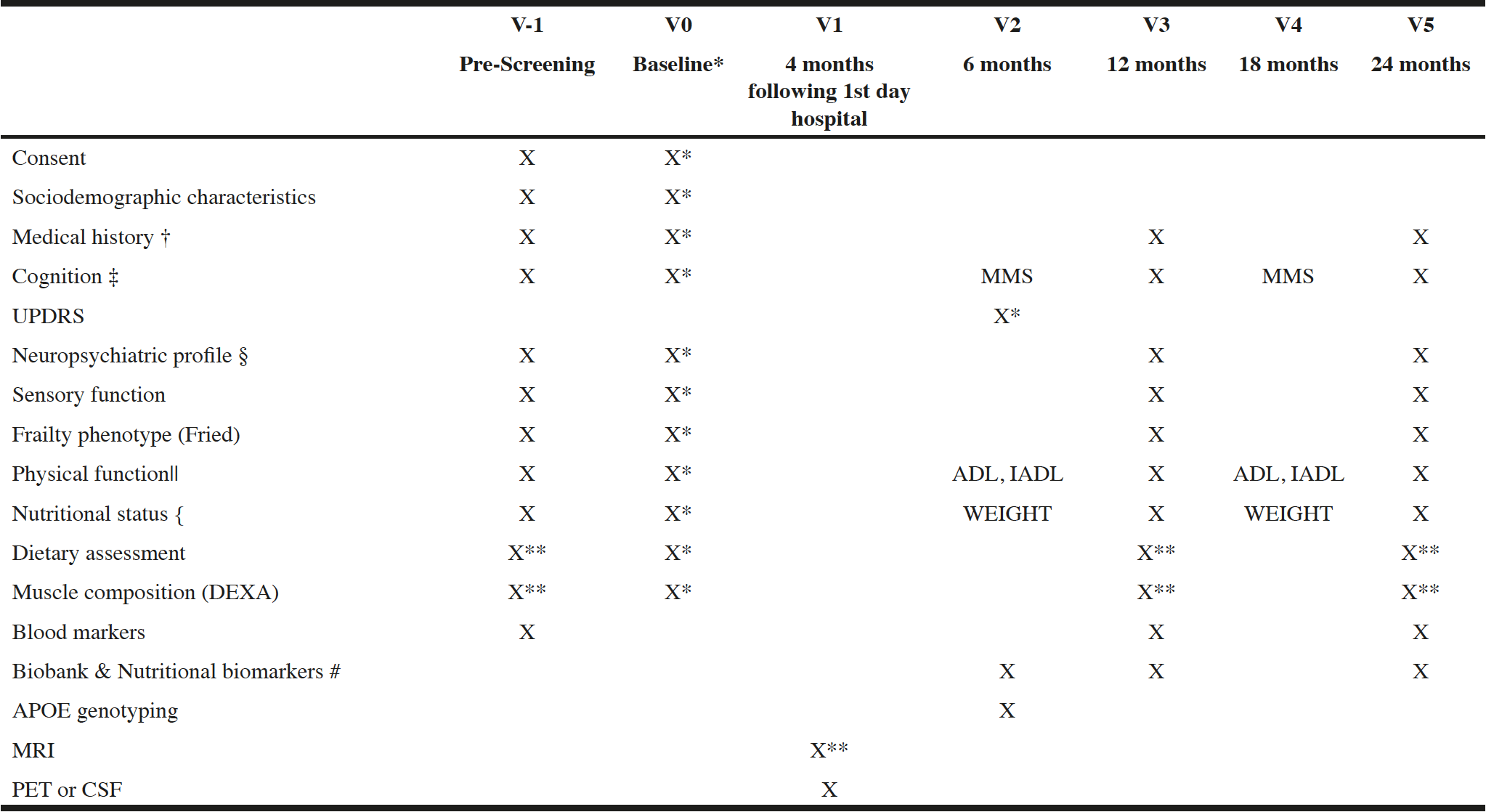
The study protocol consists of six visits (figure 1): three annual frailty assessment (baseline visit, visit at year 1 and 2), one neuroimaging visit within 4 months after inclusion, and two follow-up visits for medical consultation at 6 and 18 months.
Table 1 and 2 shows the details of study outcomes and data collection time points.
PET : Positron Emission Tomography , CSF : Cerebrospinal Fluid, MMSE : Mini-Mental State Examinatino, CDR : Clinical Dementia Rating MRI : Magnetic Resonance Imaging
* if non evaluated during screening visit; † Associated pathologies, Treatments, family history of disease; ‡ MMSE, CDR, FCSRT, DSST, COWAT, CNT, TMT; § NPI, GDS, EQ-5D-5L; || IADL, ADL, SPPB; { MNA, BMI, weight; # Fatty acid profile, Vitamin D, B6, B9, B12, homocystein; ** optional
Cognition
The Mini-mental State Examination (MMSE) and CDR scale are used to assess global cognitive impairment at baseline. For cognitive outcomes, MMSE is repeated every 6 months and CDR each year. A neuropsychological battery is performed by a neuropsychologist at baseline, 12 and 24 months and is detailed in table 1.
Four of these cognitive tests are used to create a « composite z score » previously used in MAPT study (23).
Physical function
Frailty is evaluated according to Fried phenotype, based on the five physical criteria developed by Fried and colleagues: weight loss, exhaustion, weakness, low physical activity and slowness (20). Weight loss is defined by an unintentional weight loss of more than 4.5 kg in the previous year. Self-reported exhaustion is assessed using two questions from Center for Epidemiological Studies Depression (CES-D) scale: “How often did you feel that everything you did was an effort?” and “How often did you feel that you could not get going” (24). Participants that answered “most of the time” or “often” to one of the questions were categorized as frail for this item. Weakness / hand grip strength is measured with a dynamometer: interpretation of results takes into account sex and body mass index [BMI]). Slow gait speed is defined by walking time over a distance of 4 meters: interpretation of results takes into account sex and height. Physical activity is evaluated using a scale developed by Saltin and Grimby and modified by Mattiasson-Nilo et al. (25). This Classification system of physical activity includes physical training/ exercises and domestic activities and consists of 6 grades: 1/ Hardly any physical activity, 2/ Mostly sitting, sometimes a walk, light gardening or similar tasks, sometimes light household activities such as heating up food, dusting or clearing up 3/ Light physical exercise around 2–4 h a week such as walks, fishing, dancing, and ordinary gardening, including walks to and from shops. Main responsibility for light domestic work such as cooking, clearing up and making beds. Performs or takes part inweekly cleaning, 4/ Moderate exercise 1–2 h a week, e.g., jogging, swimming, gymnastics, heavy gardening, home-repairing, or light physical activity more than 4 h a week. Responsible for all domestic activities, light as well as heavy. Weekly cleaning with vacuum cleaning, washing floors and window-cleaning, 5/ Moderate exercise at least 3 h a week, e.g., tennis, swimming, and jogging, 6/ Hard or very hard exercise regularly and several times a week, where physical exertion is great, e.g., jogging and skiing. The “low physical activity” criteria is met if the participant is graded 1 or 2. According to these criteria, subjects were categorized as non-frail (=0 criteria), pre-frail (=1 or 2 criteria), or frail (≥3 criteria) (20). Physical performance is evaluated with the Short Physical Performance Battery (SPPB) scale, a tool combining gait speed, chair stand and balance tests (26). To explore the existence of Parkinson’s signs, the Unified Parkinson’s Disease Rating scale will be evaluated at the second visit at 6 months (27).
Moreover, a dual energy X-ray absorptiometry (DEXA) is be performed optionally at baseline, 12 and 24 months (system Lunar iDXA), to measure the whole body composition (fat mass, muscle mass, bone mass) and identify sarcopenia.
Food intake and nutritional status
Nutritional status is assessed using baseline body weight, change in body weight, Body Mass Index (BMI, in kg/m2) and the Mini Nutritional Assessment-Long Form (28). Depending on total MNA score, patients are classified as well nourished (>23.5 points), at risk of malnutrition (17–23.5 points) or malnourished (<17 points).
The dietary macronutrient and micronutrient intake of the participants is assessed using diet history interviews performed annually with the clinics dietitian. Diet history is a detailed interview (lasting about 45 min) on the usual dietary intake of the subject including all meals, drinks (including alcohol consumption) and snacks. This interview will collect a typical daily intake pattern, including amount, frequencies and methods of preparation. The resulting data are then analyzed using Nutrilog software (SAS, France) to calculate the nutrient intake.
Beta amyloid-level: PET imaging and CSF amyloid level
The primary outcome of this study is the prevalence of subjects with a positive amyloid status as corroborated with amyloid Positron Emission Tomography (PET) or amyloid-β-1-42 level in CSF. Amyloid PET imaging is a relatively non-invasive technique to detect brain beta-amyloid pathology, and has proven to be effective in the early diagnosis of AD (29). To date, only a few studies have explored cerebral amyloid pathology using amyloid PET scans in frail patients (13, 14).
Participants receive an intravenous injection of the following radiotracers, depending on their availability: Amyvid (18F-Florbetapir), Neuraceq (18F-Florbetaben) or Vizamyl (18F-Flutemetamol). A systematic review and meta-analysis exploring the diagnostic accuracy of these biomarkers for AD did not find differences between these 3 amyloid radiotracers (30). The injected dose depends on the radiotracer selected: 4Mbq/kg for florbetapir, 300 Mbq for florbetapen and 185 Mbq for flutemetamol. PET Scans were acquired at the PET facility of Toulouse University Hospital using a BiographTM 6 TruePointTM (Siemens Medical Solutions, Munich, Germany) high-resolution hybrid PET/CT scanner. Following the Euratom Directive 96/29 and its implementation in France by Decree 2002-460 for healthy volunteers and patients, the injected dose and the parameters of brain scanner are adapted to a quality examination with the least irradiation possible. Image emission starts 90 (+5) minutes after radiotracer injection, for a 10 minutes acquisition period. The PET images are reconstructed iteratively and attenuation corrected according to the Hounsfield unit scale from the brain scan.
Amyloid positivity is determined by visual reading: 2 independent nuclear medicine physicians, highly trained to amyloid PET reading and blind to all clinical and diagnostic informations, review all PET scans in a randomized order. They use a binary scale to classify each scan as “amyloid negative” in absence of significant cortical retention, and “amyloid positive” in presence of significant cortical retention. In case of disagreement, the two reviewers review the images again until a consensus is reached.
Global as well as regional standardized uptake volume ratios (SUVRs) will also be obtained by semi-quantitative analyses, using the whole cerebellum as reference region. Determination of SUVRs cut-off will be determined according to the literature, and others cut-off will be explored.
Depending on the patient’s acceptablity and the clinical context, a lumbar puncture with CSF amyloid-β-1-42 measurement can be proposed, as a second option to measure beta-amyloid level. If CSF is collected, CSF level of total tau and phosphorylated tau are also measured. While not always interchangeable, PET amyloid imaging and CSF amyloid level show high correlation, with good concordance using dichotomized variables (31, 32). A lumbar puncture is performed following standardized conditions to collect 5 to 10 mL of CSF sample in 2 polypropylene tubes. The quantitative determination of beta-amyloid peptide in CSF will be performed at the Toulouse Bio Ressources (Centre de Ressources Biologique CRB) using the INNOTEST B-amyloid (1-42) enzyme immunoassay (FUJIREBIO). A cut-off of 500 pg/mL will be applied for the determination of the amyloid status.
Magnetic Resonance imaging
Only a few neuroimaging studies have investigated the association between frailty and structural neuroimaging markers (33, 34). One of the main finding was the positive association between a higher burden of white matter lesions and the prevalence of frailty but also slow gait speed. A positive association between slow gait speed and brain atrophy, smaller gray matter volume, smaller total hippocampal volume and resting state connectivity were also suggested in some studies (35–37), but these results remain scarce.
Our objective is a better understanding of brain imaging modifications associated with frailty and cognitive impairment and to identify potential biomarkers of cognitive frailty. A MRI is performed once, within 4 months of the inclusion. All MRI data are collected on a 3-Tesla MRI scanner with a multi-channel head coil. The imaging analyses rely on the CATI (a platform dedicated to multicenter neuroimaging). The protocol is partly derived from the ADNI (Alzheimer Disease Neuroimaging Initiative) study and includes a scan with the following sequences: 3DT1, T2FLAIR, T2FSE, T2GRE, resting-state functional MRI and diffusion MRI. Quality controls will rely on the CATI. MRI analysis include: whole brain volumetry (and total grey and white matter volume), hippocampal volumetry, white matter hyperintensities volume, connectivity indexes, cortical thickness, indices derived from diffusion imagin in region of interests and sulcal morphology.
This examination is not mandatory, depending on compatibility for MRI examination and medical pertinence (patients who underwent a cerebral routine MRI scan for cognitive investigation on the year preceding inclusion were not asked systematically to undergo a second examination).
Blood sampling
Blood samples are collected at 2 times point during the study (at 6 months and 24 months) to obtain longitudinal data on biomarkers. A 22 ml blood sample is taken in fasting condition each time: 2 clot activator tubes (2x 6 mL), 1 ethylenediaminetetraacetic (EDTA) tube (6 mL) and 1 lithium heparin tube (4 mL). Right after blood drawing, the samples are transferred directly at 4°C to the CRB (Centre de Ressources Biologiques) Toulouse Bio Ressources. One milliliter of blood is taken to measure immediately a series of nutritional biomarkers (Vitamin D, B6, B9, B12, homocysteine and fatty acids including EPA and DHA), due to their instability. The rest of the blood sample (plasma, serum and pellet) is aliquoted in 500 µL cryotubes and frozen at -20°C to constitute a biobank. All aliquots are in a second time transferred in a freezer at -80°C. The biobank will permit to measure a range of biological marker of aging potentially linking frailty to cognitive impairment (e.g inflammatory, metabolomics, nutritional, oxidative stress or genomic markers). A biobank scientific committee will be set up, in collaboration with the one of the INSPIRE study (38), to determine the scientific directions and research priorities in this cohort.
Additionally, results from the standard blood tests usually done in clinical routine at the Frailty or Memory Clinic at the time of inclusion are extracted from patients medical record: they include CRP, creatinine, hemoglobin, platelets, leukocytes, and TSH.
Biological ancillary studies
An ancillary study to explore the link between peripheral blood mononuclear cells (PBMC) and neurodegeneration was submitted 20 march 2020. Two additional EDTA tubes (2×6 mL) will be collected at 6, 12 and 24 months for immunological assessment. The samples will be transferred at room temperature to CRB where PBMC will be collected after density gradient-based separation, counted and frozen at 10 millions/cells per vials. Frozen vials will be stored in liquid nitrogen by the immune monitoring platform at the Center for Pathophysiology of Toulouse-Purpan (CPTP).
Health Economic
Costs examined are those related to the management of patients enrolled in the study. Cost estimates are performed from the health insurance perspective and more broadly from the societal perspective (i.e. patients and families). Costs taken into account are direct medical costs (inpatient, outpatient, and medication), direct non-medical costs (transportation, formal care) and informal costs. The expenses incurred in the management of patients are recorded over a 2 years period from the French health insurance databases and from questionnaires for formal and informal costs.
Ethical approval
Ethical approval was obtained from the institutional research committee (CPP SOOM II) on 02 December 2016 ((Registration Number: RC31/16/8753). The protocol was registered on ClinicalTrials.gov (NCT03129269).
Discussion
The COGFRAIL study will give the opportunity to determine for the first time the prevalence of cerebral amyloid pathology in frail older adults with cognitive impairment, to assess their cognitive and physical progression over a 2 years follow-up period, and to explore the impact of a range of nutritional, imaging, biological marker in the development and progression of cognitive frailty.
Many Alzheimer drug trials have failed through the last several years. We stressed that frailty and dementia could be closely interrelated, but frailty is not taken into account in AD trials and could be a new axis of intervention(39). COGFRAIL will give a unique opportunity to better understand the links between AD, aging, and frailty.
First, this study is an opportunity to better understand the links between amyloid pathology and frailty. Recently, with the perspective to explore the discrepancy between brain level of AD pathology and cognitive decline, Wallace et al. suggested that frailty could moderate the relationship between AD pathology and the expression of dementia (40). They suggested that frailty could modulate the expression of AD pathology: subjects with a low degree of frailty were less prone to express AD dementia in presence of AD pathology, and inversely subjects with a high degree of frailty are more likely to express AD dementia even with low burden of AD pathology. The COGFRAIL study could allow to assess the impact of physical frailty on brain areas involved in cognitive resilience (with brain baseline MRI) and to study brain mechanisms involved in the clinical expression of frailty and amyloid pathology.
Secondarily, this study will focus on the role of nutrition in the development and progression of cognitive frailty. There is growing evidence that vitamins B6, B12, B9, polyphenol, carotenoid and omega-3 PUFA (polyunsaturated fatty acids) may play a protective role in cognitive decline (41, 42). Deficiencies in these micronutrients are common in older adults, particularly those who are frail or cognitively impaired (43, 44). They may represent a population of interest on which nutritional strategies could be targeted to slow down neurodegenerative progression. However, certain studies have provided controversial results on the effect of dietary regimens or nutritional supplementation on cognitive decline (including study of cognitive functions, and brain imaging biomarkers). One of the potential reason is the lack of data supporting probable synergies between nutrients or food groups (45, 46) because they were focused on single nutrients. Moreover, interactions with others factors such as health behaviours, ApoE genotype, or other personal characteristics (46, 47) must be taken in account to better understand direct and indirect relationships between nutrition and cognitive decline. The COGFRAIL study will give us significant information on the impact of nutrition on cognition and physical frailty using a wide range of data including blood measurement of nutritional biomarker, detailed dietary interview, clinical assessment and DEXA measurement.
Gathering blood samples and longitudinal clinical data on cognitive and physical functions (but also mood, nutrition and sensory functions) from older persons at risk of functional decline will allow us to test geroscience hypotheses. Indeed, markers of biological age are needed to better understand the heterogeneity of the aging process among individuals, to implement interventions to promote healthy aging, and to monitor the response to these interventions. To date, putative biomarkers of aging were proposed mainly for their association(s) with mortality or age-related disease, but none of them are validated for their ability to predict the decline of several functions (e.g. cognition and locomotion) (48–50). For this purpose, we will combine « omics » and hypothesis-driven approaches focused on putative biomarkers related to hallmarks of biological aging (e.g. loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, or inflammaging) (51,52). The COGFRAIL study is associated to the Inspire Research Platform on Healhty Aging and Gerosciences, a translational geriatric program (53–55).
Conclusion
Results of the study will help characterize the cognitive decline in frail and pre-frail patients, with important implications for the identification, management and prevention of neurocognitive disorders among frail old individuals. If mechanistic frailty-cognitive decline links are established, these results may also help optimize the early identification of patients with or even at risk of AD-dementia by taking into account physical parameters that are not conventionally looked at in AD, such as frailty parameters (e.g. gait speed) or muscle composition. Such an outcome might also open a window to novel prevention and treatment strategies in AD.
Funding: The COGFRAIL study has obtained funding from MSDAVENIR.
Potential Conflicts of Interest: All authors declare to have no financial relationship with any organisations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgement: The authors thank MSD AVENIR and its support from the beginning of the study, but also all the health professionals participating in the COGFRAIL study.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet Lond Engl. 2013 Mar 2;381(9868):752–62.
2. Kiiti Borges M, Oiring de Castro Cezar N, Silva Santos Siqueira A, Yassuda M, Cesari M, Aprahamian I. The Relationship between Physical Frailty and Mild Cognitive Impairment in the Elderly: A Systematic Review. J Frailty Aging. 2019;8(4):192–7.
3. Kojima G, Liljas A, Iliffe S, Walters K. Prevalence of Frailty in Mild to Moderate Alzheimer’s Disease: A Systematic Review and Meta-analysis. Curr Alzheimer Res. 2017;14(12):1256–63.
4. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment–a review of the evidence and causal mechanisms. Ageing Res Rev. 2013 Sep;12(4):840–51.
5. Aguilar-Navarro SG, Mimenza-Alvarado AJ, Anaya-Escamilla A, Gutiérrez-Robledo LM. Frailty and Vascular Cognitive Impairment: Mechanisms Behind the Link. Rev Investig Clin Organo Hosp Enfermedades Nutr. 2016 Feb;68(1):25–32.
6. Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017 Feb;29(1):43–8.
7. Morley JE. Cognition and nutrition. Curr Opin Clin Nutr Metab Care. 2014 Jan;17(1):1–4.
8. Halil M, Cemal Kizilarslanoglu M, Emin Kuyumcu M, Yesil Y, Cruz Jentoft AJ. Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015 Mar;19(3):276–83.
9. Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008 Aug 12;71(7):499–504.
10. Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013 May 28;80(22):2055–61.
11. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014 Dec;69(12):1536–44.
12. Del Campo N, Payoux P, Djilali A, Delrieu J, Hoogendijk EO, Rolland Y, et al. Relationship of regional brain β-amyloid to gait speed. Neurology. 2016 Jan 5;86(1):36–43.
13. Yoon DH, Lee J-Y, Shin SA, Kim YK, Song W. Physical Frailty and Amyloid-β Deposits in the Brains of Older Adults with Cognitive Frailty. J Clin Med. 2018 Jul 9;7(7).
14. Maltais M, de Souto Barreto P, Hooper C, Payoux P, Rolland Y, Vellas B, et al. Association between brain β-amyloid and frailty in older adults. J Gerontol A Biol Sci Med Sci. 2019 Jan 9;
15. Wallace L, Theou O, Rockwood K, Andrew MK. Relationship between frailty and Alzheimer’s disease biomarkers: A scoping review. Alzheimers Dement Amst Neth. 2018;10:394–401.
16. Vellas B, Balardy L, Gillette-Guyonnet S, Abellan Van Kan G, Ghisolfi-Marque A, Subra J, et al. Looking for frailty in community-dwelling older persons: the Gérontopôle Frailty Screening Tool (GFST). J Nutr Health Aging. 2013 Jul;17(7):629–31.
17. Tavassoli N, Guyonnet S, Abellan Van Kan G, Sourdet S, Krams T, Soto ME, et al. Description of 1,108 older patients referred by their physician to the “Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability” at the gerontopole. J Nutr Health Aging. 2014 May;18(5):457–64.
18. De Kerimel J, Tavassoli N, Lafont C, Soto M, Pedra M, Nourhashemi F, et al. How to Manage Frail Older Adults in the Community? Proposal of a Health Promotion Program Experienced in a City of 16,638 Inhabitants in France. J Frailty Aging. 2018;7(2):120–6.
19. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry J Ment Sci. 1982 Jun;140:566–72.
20. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56.
21. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963 Sep 21;185:914–9.
22. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–98.
23. Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017 May;16(5):377–89.
24. Schroevers MJ, Sanderman R, van Sonderen E, Ranchor AV. The evaluation of the Center for Epidemiologic Studies Depression (CES-D) scale: Depressed and Positive Affect in cancer patients and healthy reference subjects. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2000;9(9):1015–29.
25. Mattiasson-Nilo I, Sonn U, Johannesson K, Gosman-Hedström G, Persson GB, Grimby G. Domestic activities and walking in the elderly: evaluation from a 30-hour heart rate recording. Aging Milan Italy. 1990 Jun;2(2):191–8.
26. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–94.
27. Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord Off J Mov Disord Soc. 2003 Jul;18(7):738–50.
28. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med. 2002 Nov;18(4):737–57.
29. Pontecorvo MJ, Mintun MA. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer’s disease. Alzheimers Res Ther. 2011 Mar 29;3(2):11.
30. Morris E, Chalkidou A, Hammers A, Peacock J, Summers J, Keevil S. Diagnostic accuracy of (18)F amyloid PET tracers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2016 Feb;43(2):374–85.
31. Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, et al. Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. J Alzheimers Dis JAD. 2014;41(3):801–7.
32. Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al., Swedish BioFINDER study group, Alzheimer’s Disease Neuroimaging Initiative. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement J Alzheimers Assoc. 2018;14(11):1470–81.
33. López-Sanz D, Suárez-Méndez I, Bernabé R, Pasquín N, Rodríguez-Mañas L, Maestú F, et al. Scoping Review of Neuroimaging Studies Investigating Frailty and Frailty Components. Front Med. 2018;5:284.
34. Maltais M, de Souto Barreto P, Moon SY, Rolland Y, Vellas B, MAPT/DSA Study Group. Prospective association of white matter hyperintensity volume and frailty in older adults. Exp Gerontol. 2019 Apr;118:51–4.
35. Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PloS One. 2014;9(1):e84909.
36. Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology. 2015 Aug;57(8):851–61.
37. Crockett RA, Hsu CL, Best JR, Liu-Ambrose T. Resting State Default Mode Network Connectivity, Dual Task Performance, Gait Speed, and Postural Sway in Older Adults with Mild Cognitive Impairment. Front Aging Neurosci. 2017;9:423.
38. De souto Barreto P, Guyonnet S, Andrieu S, Casteilla L, Davezac N, Dray C, et al. The Inspire Research Iniative: A Program for Geroscience and Health Aging Research going from Animal Models to Human and the Healthcare System. J Frailty Aging. 2020;
39. Lyreskog DM. Frailty and Neurodegenerative Disease: Anticipating the Future, Expanding the Framework. J Frailty Aging. 2018;7(1):57–9.
40. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019 Feb;18(2):177–84.
41. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J Alzheimers Dis JAD. 2017;59(3):815–49.
42. Vauzour D, Camprubi-Robles M, Miquel-Kergoat S, Andres-Lacueva C, Bánáti D, Barberger-Gateau P, et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev. 2017 May 1;35:222–40.
43. Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017 15;17(1):108.
44. De Wilde MC, Vellas B, Girault E, Yavuz AC, Sijben JW. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimers Dement N Y N. 2017 Sep;3(3):416–31.
45. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv Nutr. 2016 May 1;7(3):445–54.
46. Barberger-Gateau P, Samieri C, Féart C, Plourde M. Dietary omega 3 polyunsaturated fatty acids and Alzheimer’s disease: interaction with apolipoprotein E genotype. Curr Alzheimer Res. 2011 Aug;8(5):479–91.
47. Barberger-Gateau P. Nutrition and brain aging: how can we move ahead? Eur J Clin Nutr. 2014 Nov;68(11):1245–9.
48. Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. GeroScience. 2018;40(5-6):419–36.
49. Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. EBioMedicine. 2017 Jul;21:29–36.
50. Guerville F, De Souto Barreto P, Ader I, Andrieu S, Casteilla L, Dray C, et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J Prev Alzheimers Dis. 2020;7(1):56–64.
51. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194–217.
52. Mostafavi S, Gaiteri C, Sullivan SE, White CC, Tasaki S, Xu J, et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat Neurosci. 2018;21(6):811–9.
53. Takeda C, Guyonnet S, Sumi Y, Vellas B. Integrated Care for Older People and the Implementation in the INSPIRE Care Cohort. J Prev ALz Dis. 2020;2(7):70–4.
54. Tavassoli N, PIau A, Berbon C, de Kerimel J, Lafont C. Framework implementation of the INSPIRE ICOPE-CARE Program in collaboration with the World Health Organization (WHO) in the Occitania Region. J Frailty Aging. DOI:10.14283/jfa.2020.26
55. Sierra F. Editorial: Geroscience and the Role of Aging in the Etiology and Management of Alzheimer’s Disease. J Prev Alzheimers Dis. 2020;7(1):2–3.
56. Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988 Jun;38(6):900–3.
57. Wechsler D. Wechsler adult intelligence scale-revised. Psychological Corp. New York; 1981.
58. Reitan R. Validity of the Trail Making Test as an indicator of brain damage. Percept Mot Ski. 1958;8:271–6.
59. Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. [Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level]. Acta Neurol Belg. 1990;90(4):207–17.
60. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982 1983;17(1):37–49.
61. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–86.
62. Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3–11; discussion 11–2.