A.K. Stuck1, A. Weber2, R. Wittwer1, A. Limacher3, R.W. Kressig4
1. Department of Geriatrics, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland; 2. University of Bern, Medical Faculty, Bern, Switzerland; 3. CTU Bern, University of Bern, Bern, Switzerland; 4. University Department of Geriatric Medicine FELIX PLATTER, Basel, and University of Basel, Basel, Switzerland
Corresponding Author: Dr. med. Anna K. Stuck, Department of Geriatrics, Inselspital, Bern University Hospital, and University of Bern, Freiburgstrasse 46, CH-3010 Bern, Switzerland, e-Mail: anna.stuck@insel.ch
J Frailty Aging 2022;11(2)156-162
Published online October 1, 2021, http://dx.doi.org/10.14283/jfa.2021.40
Abstract
Objectives: To investigate practicality and repeatability of a handheld compared to a state-of-the-art multisegmental bioelectrical impedance analysis (BIA) device to facilitate screening of sarcopenia in older inpatients.
Design and setting: Cross-sectional study in a geriatric rehabilitation hospital.
Participants: 207 inpatients aged 70+.
Measurements: In a first phase, appendicular skeletal muscle mass index (ASMI) was measured using the handheld Biody xpertZm II BIA device (n=100). In a second phase, ASMI was obtained using the multisegmental Biacorpus RX 4004M device (n=107). Repeatability of BIA devices was compared in subgroups of patients (handheld BIA device: n=36, multisegmental BIA device: n=46) by intra-class correlation (ICC) and Bland-Altman plots.
Results: Overall, measurement failure was seen in 31 patients (31%) tested with the handheld BIA device compared to one patient (0.9%) using the multisegmental BIA device (p<0.001). Main reasons for measurement failure were inability of patients to adopt the position necessary to use the handheld BIA device and device failure. The mean difference of two ASMI measurements in the same patient was 0.32 (sd 0.85) using the handheld BIA device compared to 0.02 kg/m2 (sd 0.07) using the multisegmental device (adjusted mean difference between both groups -0.35, 95% confidence interval (CI) -0.61 to -0.09 kg/m2). Congruently, Bland-Altman plots showed poor agreement with the handheld compared to the multisegmental BIA device.
Conclusion: The handheld BIA device is neither a practical nor reliable device for assessing muscle mass in older rehabilitation inpatients.
Key words: Appendicular skeletal muscle mass, sarcopenia, repeatability, practicality, bioelectrical impedance analysis, geriatric.
Introduction
Sarcopenia is a common disease primarily affecting older and multimorbid patients (1) and is associated with poor outcomes, such as falls and mortality (2, 3). It is important to identify older patients presenting with sarcopenia in order to begin appropriate targeted interventions (4). According to the most recent guidelines by the European Working group on sarcopenia (EWGSOP2), muscle mass, together with muscle strength, are considered the two criteria necessary to diagnose sarcopenia (5).
Currently, bioelectrical impedance analysis (BIA) using a multisegmental device is considered the portable reference method for measuring muscle mass. Thereby, mutisegmental BIA devices are commonly used in inpatients in the screening work-up of sarcopenia, osteosarcopenia, malnutrition, cachexia, obesity, and frailty (6-11). In older inpatients, validity of multisegmental BIA devices to measure muscle is acknowledged (12, 13).
However, multisegmental BIA devices are typically large, heavy, and require installation on a mobile cart. From a clinical perspective, a handheld pocket device would be more ideal in geriatric inpatient institutions (e.g. geriatric acute and post-acute hospitals, nursing homes), and could eventually facilitate routine screening of sarcopenia in older patients.
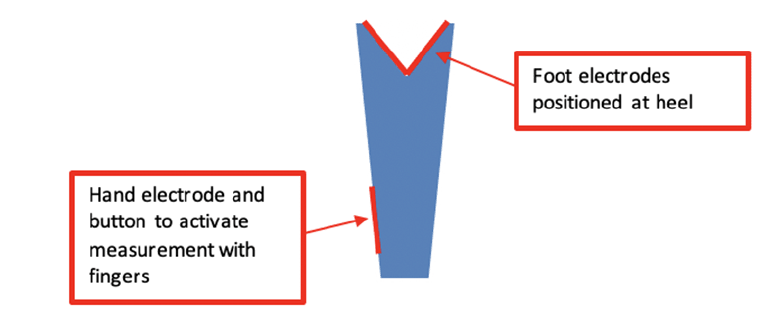
A promising handheld pocket BIA device (BIODY XPERTZM II) has recently become available for bedside evaluation of muscle mass (Figure 1). While potentially a useful tool for diagnosing sarcopenia, we were unable to find data demonstrating how this device performs in the assessment of older inpatients.
This study evaluated the practicality and repeatability of this new handheld BIA device compared to a multisegmental BIA device among older patients in a geriatric inpatient facility.
Methods
Setting
We conducted a retrospective analysis of cross-sectional anonymized assessment data of all patients admitted to a Geriatric Rehabilitation Hospital in Bern Switzerland, between September and December 2019 (n=207). All patients met the following admission criteria: (1) age>75 years, (2) direct transfer from acute care hospital, (3) living in the community (i.e., not in a nursing home) prior to acute care hospital admission, (4) potential for functional improvement and discharge home following inpatient rehabilitation. No patient data were excluded for this analysis.
Standard geriatric assessment was performed by designated clinically trained assessors upon admission. The assessment validated for older persons included a mini-mental status test examination (MMSE) (14) (cognitive deficit ≤26 points), clock-test (15) (deficit in executive and visuospatial functions ≤5 points), 5-item geriatric depression scale (GDS-5) (16) (depressive symptoms ≥2 points), nutritional risk screening 2002 (17) (nutritional deficit ≥3 points), and vision (18) and hearing impairment testing (19). Gait speed was measured using a standardized protocol (20) (low gait speed ≤0.8m/sec), and frailty was assessed using the clinical frailty scale (21) (frailty ≥5 points). According to recommendation by the “Deutsche Gesellschaft für Geriatrie” we used German versions of the referenced assessments (22). Body weight and height were measured using standard methods and BMI was calculated.
The study was approved by the Ethics committee “Kantonale Ethikkomission Bern”, Switzerland (Req-2020-00125).
Bioelectrical Impedance Analysis
Measurement of muscle mass was also part of the admission geriatric assessment, unless there was an absolute contraindication (i.e, patient had a pacemaker or internal cardioverter defibrillator because of an increased arrhythmic potential on implanted electronic devices). The handheld BIA device was used to measure muscle mass of all patients admitted during time period 1 (September 23 to November 11, 2019) (n=100). The multisegmental BIA device was used for patients admitted during time period 2 (November 11 to December 9, 2019) (n=107). Assessments of muscle mass were performed and monitored according to manufacturer’s guidelines and are described in detail in the following paragraphs.
Handheld BIA device
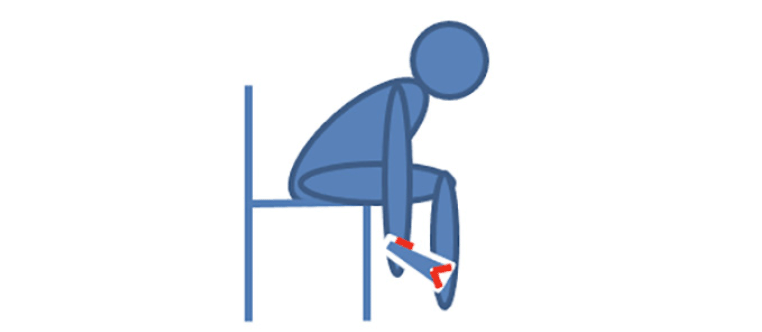
The BIA-device BIODY XPERTZM II is a unilateral validated handheld pocket device to measure muscle mass with software that uses the appendicular skeletal muscle mass index (ASMI) formula developed by Ursula Kyle (7). The device is designed for optimal testing to be conducted on the right side of the body with the patient in a seated position. The hand and heel were moisturized with a disinfecting standardized towel and patients were asked to remove all jewelry. The patient was instructed to lean over and place the device against their right heel and push the button on the side of the device. Patients were also tested in a supine position if they were unable to safely perform the test in a seated position. Patients with right-side prostheses, an internal metal device, or clinical asymmetry of body sides (e.g., right hemiplegia with muscle atrophy) were tested on the left side. During testing, the assessor insured that the patient did not touch any metal (e.g. chair leg, or bedrail) and that the two sides of the body did not come into contact. If necessary, the assessor provided assistance, and pressed the release button at the thumb electrode wearing isolating rubber gloves.
Multisegmental device
The multisegmental BIA device that we used during the second testing period was the BIACORPUS RX 4004M. The integrated software of the BIA device calculates the ASMI based on the Sergi equation (23, 24). Measurements were performed in a supine position in a standard hospital bed (maximum 30° inclination of the head). The patient rested in a supine position for 5 minutes before measurements were initiated. Both hands and heels were moisturized using a disinfecting standard towel. Two electrodes were placed at each extremity. The upper border of the proximal electrode was placed on the imaginary line between radius and ulna head, and between medial and lateral malleoli, respectively. Distal electrodes were put on within a 5cm distance of the distal border of the proximal electrodes. Whenever possible, measurement was performed bilaterally on both sides. Unilateral measurement (right or left body side) was only performed if the patient had prostheses or internal metal parts in one body side, or if the patient had unilateral atrophy or amputation. The patient was allowed to wear jewelry provided the electrodes were not blocked. As with the handheld device, the assessor insured that the patient did not touch any metal and that the two sides of the body did not come into contact. Mobile telephones had to be placed at least 1m from the measuring device.
Practicality of BIA-devices
To assess the practicality of using each device in a geriatric inpatient setting, the assessor recorded the total number of attempts that were necessary to achieve a valid measurement, any alterations in the testing procedure that were necessary to achieve a valid measurement (e.g., supine vs sitting position, assessor assistance), reasons for test failure and contraindications for use. The major reasons for test failure were categorized post-hoc into the following domains:
– Transmission error of the BIA device after 5 measurement attempts (device failure)
– Musculoskeletal impairment (e.g., limited flexibility of the hip to lean over)
– Cognitive impairment (e.g., patient unable to follow instructions)
Repeatability of BIA-devices
Repeatability was measured in a priori defined subgroup of 36 patients tested with the handheld BIA device and 46 patients tested with the multisegmental BIA device. For each patient, two consecutive measurements were performed by the same assessor using the same BIA device. Repeatability was defined according to the definition by Bartlett et al. (25).
Statistical Analysis
Study sample characteristics from admission data are presented by absolute and relative frequencies or by mean with standard deviation (sd) for categorical and continuous variables, respectively. Categorical variables (practicality) were compared between the handheld and the multisegmental device using chi-squared test and continuous variables (muscle mass) were compared using the Student’s t-test. Measures of repeatability (within and between patient standard deviation and intra-class correlation (ICC)) for the two BIA devices were calculated using one-way analysis-of-variance (ANOVA) models (checked for normality by visual inspection of histograms). Bland-Altman plots were generated displaying the differences between measurements 1 and 2 of ASMI against the mean of the two ASMI measurements. Repeatability coefficients were calculated as 1.96x the standard deviation of the mean difference for each BIA device (25). For analysis of descriptive results of ASMI, all patients who had a BIA measurement yielding an ASMI value were included in the secondary analysis. If a patient had two ASMI results, the lower value of the two was included for descriptive analysis. Linear regression analysis was performed to compare ASMI values between the two groups using the handheld or multisegmental BIA device adjusted for age, sex and frailty status. An a priori decision was made to not perform statistical comparisons among subgroups of patients to avoid type I and II error inflation. Analyses were computed using Stata Version 16.1 (StataCorp LLC, College Station, Texas, USA). A p-value of <0.05 was considered statistically significant.
Results
The mean age of the sample was 84.3 years (standard deviation (sd) 6.4) and 65.7% were female. Descriptive characteristics of patients (n=207) are shown in Table 1. Clinical characteristics of patients measured with the handheld BIA device did not differ from characteristics of patients measured with the multisegmental device.
Abbreviations: BIA, bioelectrical impedance analysis; sd, standard deviation; MMS, mini mental status examination; NRS, nutritional risk score; GDS, 5-item geriatric depression scale; BMI, body mass index; a. No data due to inability to perform clock-test in n=18 (handheld BIA), and n=14 (multisegmental); b. No data due to cognitive impairment in n=7 (handheld BIA), and n=14 (multisegmental BIA); c. Missing data in n=1 (handheld BIA); d. P-value indicated for the comparison of patients with handheld BIA vs. patients with multisegmental BIA.
Practicality
Overall, measurement of muscle mass was not possible in 36 (36%) of the 100 patients with the handheld, and in 9 (8.4%) with the multisegmental device, respectively. This was in part due to a contraindication for BIA measurement (see Methods section for list of contraindications), and in part due to an inability to obtain a measurement value (Table 2). In specific, five patients had a contraindication for use of the handheld BIA device and 8 patients had a contraindication for use of the multisegmental device, but this difference was not statistically significant (5.0% vs. 7.5%, p=0.46) (Table 2). ASMI measurement was unsuccessful in 31 patients (31%) using the handheld BIA and one patient (0.9%) using the multisegmental BIA corresponding to a difference between the groups of 30.1% (95% CI, 20.9 to 39.8%; p<0.001). Reasons for inability to obtain an ASMI measurement with the handheld BIA device, other than a contraindication, included musculoskeletal impairment (n=17), transmission error of the BIA device after five measurement attempts (n=13), and cognitive impairment (n=1). In contrast, the reason for inability to obtain an ASMI result with the multisegmental device in the one patient was due to musculoskeletal impairment.
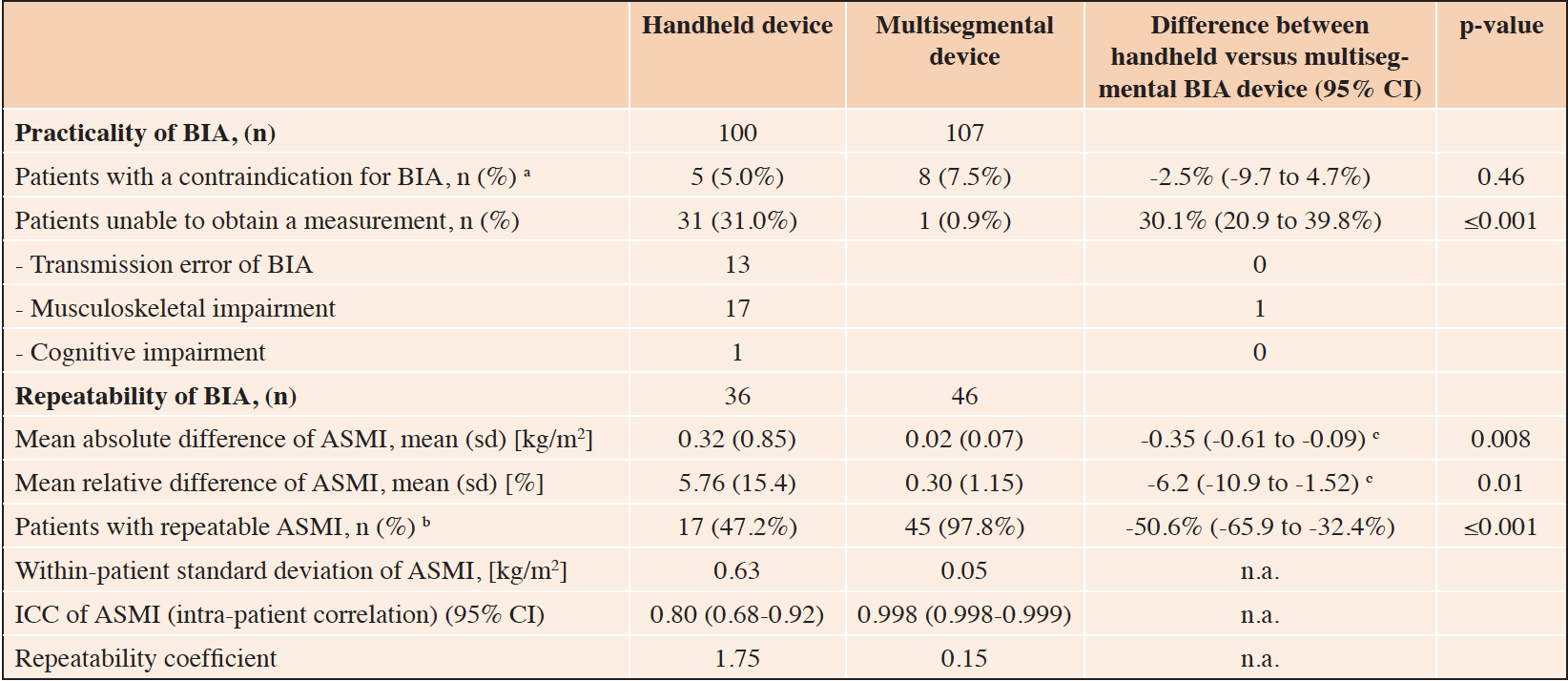
Table 2. Comparison of practicality and repeatability between the handheld and multisegmental BIA-device
Abbreviations: BIA, bioelectrical impedance analysis; sd, standard deviation; ASMI, appendicular skeletal muscle mass index; ICC, intraclass correlation coefficient; n.a., not applicable; CI, confidence interval; a. Contraindication of BIA include patients with pacemaker or an implantable cardioverter defibrillator (ICD); b. Patients with repeatable ASMI is defined as patients with two ASMI results and thereof a mean relative difference of <2.5%; c. Difference in mean difference of ASMI measurement 1 and 2 between both devices, adjusted for age, sex and frailty status using linear regression analysis
Repeatability
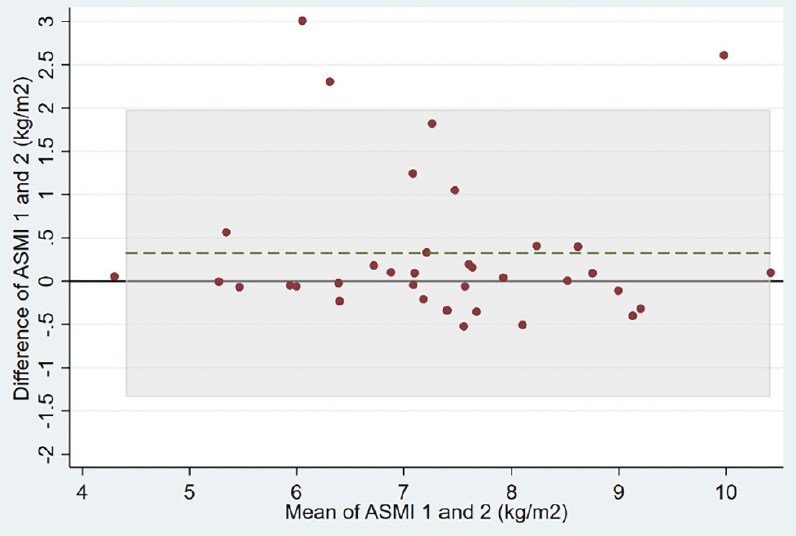
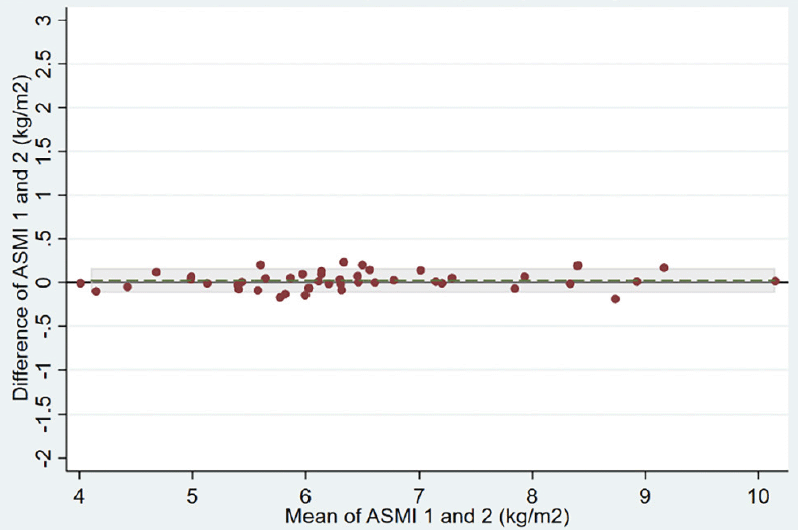
Repeatability of results for each BIA device is shown in Table 2. The ASMI mean absolute difference between two measurements of the same patient was 0.32 (0.85) kg/m2 in the group using the handheld BIA device versus 0.02 (0.07) kg/m2 in the group using the multisegmental BIA device (adjusted ASMI mean difference between the two groups of -0.35, 95% confidence interval (CI) -0.61 to -0.09 kg/m2). The variability of the two handheld BIA measurements within patients was much higher than the two multisegemental BIA measurements (within-patient standard deviation of 0.63 versus 0.05 kg/m2, respectively). Correspondingly, the intra-patient correlation was much lower for the handheld BIA device than for the multisegmental BIA device (ICC of 0.80 versus 0.998, respectively). Bland-Altman plots of the two devices also show a lower agreement for the handheld BIA device compared to the multisegmental device (Figure 2, Panels A and B).
Abbreviations: ASMI, appendicular skeletal muscle mass index; BIA, bioelectrical impedance analysis; The grey horizontal line displays the reference line indicating a difference of 0 kg/m2 between measurement 1 and 2 of ASMI. The dashed green line represents the mean difference between measurement 1 and 2 of ASMI. Shaded area represent the area within the limits of agreement defined as the mean difference ± 1.96 SD of differences.
Validity
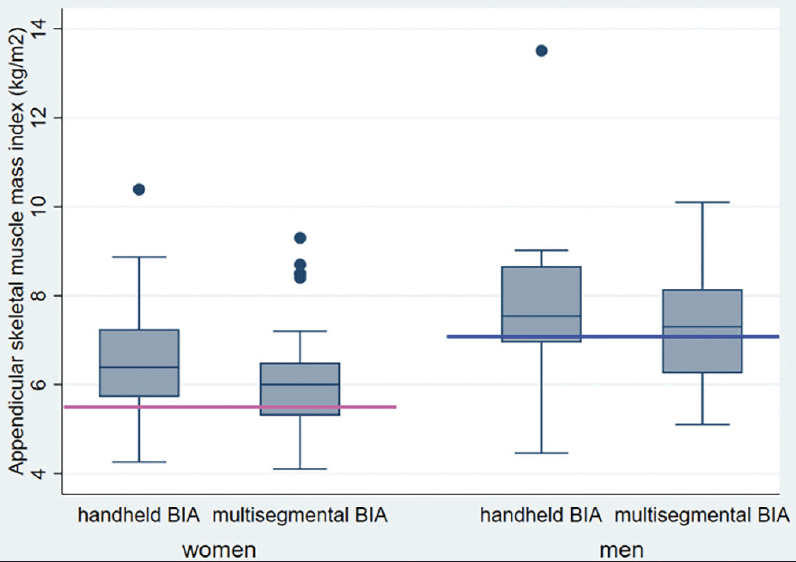
ASMI means differed significantly between patients measured with the handheld BIA and the multisegmental BIA device (6.9 (1.5) vs. 6.4 (1.3) kg/m2, respectively; mean adjusted difference, 0.51, 95% CI 0.12 to 0.90 kg/m2, p-value = 0.01). Among women, mean ASMI was 6.5 (1.3) kg/m2 using the handheld device and 6.0 (1.1) kg/m2 using the multisegmental device (Figure 3). Among men, mean ASMI was 7.7 (1.2) kg/m2 using the handheld device and 7.3 (1.2) kg/m2 using the multisegmental device.
Abbreviations: ASMI, appendicular skeletal muscle mass index; Horizontal lines indicate gender-specific minimum threshold lines of appendicular skeletal muscle mass index (ASMI) according to the European guidelines on diagnosis of sarcopenia (pink line: women=<5.5kg/m2; blue line: men =<7.0kg/m2).
Discussion
To our knowledge, this is the first study that has evaluated a handheld BIA device in geriatric inpatients. Our findings demonstrated that the handheld BIA device tested was less practical to use and had significantly lower repeatability than the multisegmental BIA device for measuring muscle mass in older inpatients of a geriatric rehabilitation hospital.
Overall, measurement failure of the handheld BIA device occurred in a large proportion of patients compared to a negligible proportion using the multisegmental BIA device. This lack of practicality of the handheld device was observed although two clinical assessors received standard instruction and training prior to using the devices. Moreover, to account for intermittent transmission errors, we allowed five measurement trials using the BIA device. We assume that in clinical practice rates of measurement failure might even be higher since training of assessors and allowance for repetition of measurements may vary.
Proof of successful application is the basic requirement for eventual implementation of a diagnostic tool. While the handheld BIA device is smaller and more portable than the multisegmental BIA device, we observed that its design contributed to measurement failures in nearly one-third of patients. The handheld BIA device requires that the patient have basic flexion of the hip and knee and minimal grip and finger strength to hold the device and to push the activation button. Activation and handling of the BIA device also requires basic cognitive performance to follow instructions. However, both these basic requirements on mobility and cognition are frequently lacking among geriatric inpatients. In contrast, the multisegmental BIA tests patients in a supine position without requiring the patient to manually activate the device, which likely contributed to the low failure rate.
Our results also reveal that the handheld device had much lower repeatability compared to the multisegmental BIA device indicating limited bias of the handheld device. Although prior evidence suggests that the handheld BIA is feasible and repeatable in younger and healthy participants (26) these results cannot be confirmed in our study of older inpatients. The most likely explanation for this finding is that the handheld device is susceptible to small changes of the patient’s position because clear reference points are lacking for the two electrodes. In contrast, standard reference points are provided for placement of the eight electrodes used with the multisegmental BIA device. Therefore, it may be more challenging to identify and maintain the correct position for ASMI measurement using the handheld device for functionally impaired older inpatients.
In our study, we found a mean ASMI of 6.4 kg/m2 using the state-of-the-art multisegmental BIA device which is consistent with previous studies of older inpatients [13, 14]. Prior findings of ASMI in a geriatric rehabilitation hospital reported a mean ASMI of 6.4 kg/m2 [13], while another study in institutionalized patients reported an ASMI of 6.3 kg/m2 (27). Additionally, van Ancum et al. observed in acute inpatients an ASMI of 7.6 kg/m2 in men and 6.5 in women that are close to our results (28).
Overall, our results from subanalyses further indicate that there may be an additional issue of limited validity of the handheld BIA device, although direct comparison between the handheld and multisegmental devices within a patient was not possible due to our study design. However, other studies similarly reported that there are differences between BIA devices suggesting that BIA devices are not necessarily interchangeable. Beaudart et al. found that the prevalence of low muscle mass and sarcopenia was dependent on the diagnostic tool used (29). In another study, Lahav et al. found that the InBody™ BIA device underestimated body fat to a higher degree then the Seca™ BIA device in both genders and in all BMI categories (30). Similarly, Kreissl et al. (31) found that in a pediatric population, the Tanita™ BIA device underestimated fat free mass compared to the Biacorpus™ BIA device used in our study.
There are several limitations to our study. First, we investigated a single handheld BIA device in one sample of older inpatients in a geriatric rehabilitation hospital. Consequently, our results may not be generalizable to other devices or populations. Second, we chose a pragmatic sequential study design, so direct comparison of BIA devices (handheld vs. multisegmental) was not possible in the same patient. We therefore adjusted differences between both groups for potential confounding variables. Nevertheless, our main findings of limited practicality and repeatability of the handheld BIA device are independent of group comparisons and would not alter our findings.
Our findings have implications both for clinical research and practice.
Further research is needed to identify a practical and valid handheld device to measure muscle mass in older inpatients to facilitate routine diagnostic work-up of sarcopenia (32). According to the latest guidelines by the European Working group on Sarcopenia Project 2 (EWGSOP 2) sarcopenia is defined as the combination of low muscle mass and low muscle strength highlighting the importance of measuring muscle mass in this vulnerable population. The key role of identifying low muscle mass is based on longitudinal evidence, that both muscle mass and strength are predictive for significant adverse outcome such as falls (33).
However, the method of bioelectrical impedance analysis itself to measure muscle mass has intrinsic limitations, due to the BIA’s absolute contraindication in patients with an internal pacemaker. Recently, pocket handheld devices using a different technology than BIA are being considered, including ultrasound (34, 35). Additional studies are needed to identify and evaluate methodological approaches with user-friendly benefits for clinical use that could promote rapid screening of muscle mass facilitating diagnosis of sarcopenia.
In conclusion, the handheld BIA device that we evaluated failed practicality and repeatability and cannot be recommended for the use in older inpatients.
Ethical Standards: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics committee “Kantonale Ethikkomission Bern”, Switzerland (Req-2020-00125).
Acknowledgements: We thank Karen R. Josephson for editing the manuscript.
Funding: This work was supported by the Forschungsfonds Geriatrie, Bern, Switzerland. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: The authors declare no conflict of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Pacifico J, Geerlings MAJ, Reijnierse EM, et al. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Experimental gerontology 2020; 131: 110801. doi:10.1016/j.exger.2019.110801.
2. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PloS one 2017; 12: e0169548. doi:10.1371/journal.pone.0169548.
3. Zhao Y, Zhang Y, Hao Q, Ge M, Dong B. Sarcopenia and hospital-related outcomes in the old people: a systematic review and meta-analysis. Aging clinical and experimental research 2019; 31: 5-14. doi:10.1007/s40520-018-0931-z.
4. Lee SY, Tung HH, Liu CY, Chen LK. Physical activity and sarcopenia in the geriatric population: A systematic review. Journal of the American Medical Directors Association 2018; 19: 378-383. doi:10.1016/j.jamda.2018.02.003.
5. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and ageing 2019; 48: 601. doi:10.1093/ageing/afz046.
6. Player EL, Morris P, Thomas T, et al. Bioelectrical impedance analysis (BIA)-derived phase angle (PA) is a practical aid to nutritional assessment in hospital in-patients. Clinical nutrition 2019; 38: 1700-1706. doi:10.1016/j.clnu.2018.08.003.
7. Hirose S, Nakajima T, Nozawa N, et al. Phase angle as an indicator of sarcopenia, malnutrition, and cachexia in inpatients with cardiovascular diseases. Journal of clinical medicine 2020; 9, doi:10.3390/jcm9082554.
8. Lunt E, Ong T, Gordon AL, Greenhaff PL, Gladman JRF. The clinical usefulness of muscle mass and strength measures in older people: a systematic review. Age and ageing 2021; 50: 88-95. doi:10.1093/ageing/afaa123.
9. Brunani A, Perna S, Soranna D, et al. Body composition assessment using bioelectrical impedance analysis (BIA) in a wide cohort of patients affected with mild to severe obesity. Clinical nutrition 2021; 40: 3973-3981. doi:10.1016/j.clnu.2021.04.033.
10. Makizako H, Kubozono T, Kiyama R, et al. Associations of social frailty with loss of muscle mass and muscle weakness among community-dwelling older adults. Geriatrics & gerontology international 2019; 19: 76-80. doi:10.1111/ggi.13571.
11. Taniguchi Y, Makizako H, Kiyama R, et al. The association between osteoporosis and grip strength and skeletal muscle mass in community-dwelling older women. International journal of environmental research and public health 2019; 16, doi:10.3390/ijerph16071228.
12. Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. Journal of the American Medical Directors Association 2014; 15: 267-272. doi:10.1016/j.jamda.2013.11.027.
13. Bertschi D, Kiss CM, Beerli N, Kressig RW. Sarcopenia in hospitalized geriatric patients: insights into prevalence and associated parameters using new EWGSOP2 guidelines. European journal of clinical nutrition 2020, doi:10.1038/s41430-020-00780-7.
14. Folstein MF, Folstein SE, McHugh PR. «Mini-mental state». A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 1975; 12: 189-198. doi:10.1016/0022-3956(75)90026-6.
15. Freedman M, Leach L, Kaplan E, et al. Clock drawing: a neuropsychological analysis. Oxford University Press, Inc., 1994. New York
16. Hoyl MT, Alessi CA, Harker JO, et al. Development and testing of a five-item version of the Geriatric Depression Scale. Journal of the American Geriatrics Society 1999; 47: 873-878. doi:10.1111/j.1532-5415.1999.tb03848.x.
17. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc EWG. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clinical nutrition 2003; 22: 321-336. doi:10.1016/s0261-5614(02)00214-5.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. The New England journal of medicine 1999; 340: 669-676. doi:10.1056/NEJM199903043400901.
19. Bagai A, Thavendiranathan P, Detsky AS. Does this patient have hearing impairment? Jama 2006; 295: 416-428. doi:10.1001/jama.295.4.416.
20. Stuck AK, Bachmann M, Fullemann P, Josephson KR, Stuck AE. Effect of testing procedures on gait speed measurement: A systematic review. PloS one 2020; 15: e0234200. doi:10.1371/journal.pone.0234200.
21. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 2005; 173: 489-495. doi:10.1503/cmaj.050051.
22. Deutsche Gesellschaft für Geriatrie. S1-Leitlinie „Geriatrisches Assessment der Stufe 2“. 2019. https://www.awmf.org/leitlinien/detail/ll/084-002.html. Accessed 22.07.2021.
23. Brun JF, Guiraudou M, Mardemootoo C, et al. Validation of the measurement of segmental body composition compared to DEXA: Interest of the measurement of trunk fat mass. Science & Sports 2013; 158-162.
24. Sergi G, De Rui M, Veronese N, et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clinical nutrition 2015; 34: 667-673. doi:10.1016/j.clnu.2014.07.010.
25. Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2008; 31: 466-475. doi:10.1002/uog.5256.
26. Pereira B. Etude de concordance impédancemètrie. Mesure de la composition corporelle à l’aide de l’impédancemètre Biody Xpert: comparaison avec la méthode d’absorptiométrie biphotonique (DXA). 2015. DRCI – CHU Clermont-Ferrand
27. Cebria IIMA, Arnal-Gomez A, Tortosa-Chulia MA, et al. Functional and clinical characteristics for predicting sarcopenia in institutionalised older adults: Identifying tools for clinical screening. International journal of environmental research and public health 2020; 17, doi:10.3390/ijerph17124483.
28. Van Ancum JM, Alcazar J, Meskers CGM, et al. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Archives of gerontology and geriatrics 2020; 90: 104125. doi:10.1016/j.archger.2020.104125.
29. Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Experimental gerontology 2015; 61: 31-37. doi:10.1016/j.exger.2014.11.014.
30. Lahav Y, Goldstein N, Gepner Y. Comparison of body composition assessment across body mass index categories by two multifrequency bioelectrical impedance analysis devices and dual-energy X-ray absorptiometry in clinical settings. European journal of clinical nutrition 2021, doi:10.1038/s41430-020-00839-5.
31. Kreissl A, Jorda A, Truschner K, Skacel G, Greber-Platzer S. Clinically relevant body composition methods for obese pediatric patients. BMC pediatrics 2019; 19: 84. doi:10.1186/s12887-019-1454-2.
32. Bauer J, Morley JE, Schols A, et al. Sarcopenia: A time for action. An SCWD position paper. Journal of cachexia, sarcopenia and muscle 2019; 10: 956-961. doi:10.1002/jcsm.12483.
33. Van Ancum JM, Pijnappels M, Jonkman NH, et al. Muscle mass and muscle strength are associated with pre- and post-hospitalization falls in older male inpatients: a longitudinal cohort study. BMC geriatrics 2018; 18: 116. doi:10.1186/s12877-018-0812-5.
34. Buckinx F, Landi F, Cesari M, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. Journal of cachexia, sarcopenia and muscle 2018; 9: 269-278. doi:10.1002/jcsm.12268.
35. Isaka M, Sugimoto K, Yasunobe Y, et al. The usefulness of an alternative diagnostic method for sarcopenia using thickness and echo intensity of lower leg muscles in older males. Journal of the American Medical Directors Association 2019; 20: 1185 e1181-1185 e1188. doi:10.1016/j.jamda.2019.01.152.