R. Otsuka1, S. Zhang1, C. Tange1, Y. Nishita1, M. Tomida1, K. Kinoshita2, Y. Kato1,3, F. Ando1,3, H. Shimokata1,4, H. Arai5
1. Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, Aichi, Japan; 2. Department of Frailty Research, Research Institute, National Center for Geriatrics and Gerontology, Aichi, Japan; 3. Faculty of Health and Medical Sciences, Aichi Shukutoku University, Aichi, Japan; 4. Graduate School of Nutritional Sciences, Nagoya University of Arts and Sciences, Aichi, Japan; 5. National Center for Geriatrics and Gerontology, Aichi, Japan.
Corresponding Author: Rei Otsuka, Department of Epidemiology of Aging, Research Institute, National Center for Geriatrics and Gerontology, 7-430 Morioka-cho, Obu, Aichi 474-8511, Japan, E-mail: otsuka@ncgg.go.jp, Tel: +81-562-46-2311
J Frailty Aging 2021;in press
Published online October 7, 2021, http://dx.doi.org/10.14283/jfa.2021.42
Abstract
Background: Frailty is a dynamic process, with frequent transitions between frailty, prefrailty, and robust statuses over time. The effect of dietary intake on frailty transitions is unknown.
Objective: To examine the association between dietary intake and frailty transitions.
Design: Survey-based retrospective analysis of the National Institute for Longevity Sciences-Longitudinal Study of Aging data.
Setting: Areas neighboring the National Center for Geriatrics and Gerontology in Aichi Prefecture, Japan.
Participants: We included 469 prefrail community dwellers aged 60–87 years who participated both in the baseline (2008–2010) and 2-year follow-up (2010–2012) surveys of the National Institute for Longevity Sciences-Longitudinal Study of Aging.
Measurements: Transitions of frailty were categorized by changes in status from baseline to follow-up: “deterioration (prefrail to frail),” “persistence (persistent prefrail),” and “reversal (prefrail to robust).” Estimated dietary (nutrients and food) intakes assessed by 3-day dietary records in each frailty transition were analyzed with a multivariate-adjusted general linear model after adjusting for sex, age, education, family income, smoking, and chronic disease.
Results: At the 2-year follow-up, 28%, 7%, and 65% of participants had robust, frail, and pre-frail status, respectively. Among 13 food groups, only milk and dairy product intake was positively associated with frailty reversal even after adjusting for all frailty criteria at baseline. Despite insignificant differences in the estimated mean intakes, the baseline intake of saturated fatty acids, potassium, and vitamin B1 tended to be the highest in the reversal group. The estimated mean (standard error) for milk and dairy product intake (g/day) was 79.1 (28.6), 129.3 (19.9), and 161.7 (21.7) for the deterioration, persistence, and reversal groups, respectively (P=0.0036, P-trend=0.0019).
Conclusions: Daily consumption of dairy products may contribute to frailty reversal and frailty prevention among older community dwellers who consume small amounts of dairy products. Other food groups showed no association with frailty status transitions.
Key words: Frailty, reversibility, diet, retrospective study.
Introduction
Frailty is an emerging global burden among the older population (1) and is characterized by a decline in functioning across multiple physiological systems that is accompanied by an increased vulnerability to stressors. Frailty is a dynamic process, with frequent transitions between frailty, prefrailty, and robust statuses over time. As an intermediate state between robust and frailty, those with prefrailty have a high risk of progressing to frailty (2, 3). Pre-frailty naturally tends to rapidly worsen and transit to frailty. However, previous studies have shown that prefrail individuals may reverse toward a robust state if appropriate interventions are taken (for example, improving nutritional intake or increasing physical activity) (4-7). Considering the high prevalence (approximately 50%) of prefrail individuals in the older population (8, 9), identifying potentially modifiable risk factors for prefrail transitions is needed.
Review studies have shown that dietary factors, such as macronutrients (e.g., protein), micronutrients [e.g., carotenoids and 25(OH)D], food groups (e.g., low-fat dairy products), and dietary patterns (e.g., the Mediterranean diet), are related to frailty syndrome or frail criteria (10-12). However, the subjects of these studies were predominantly non-frail individuals. Meanwhile, interventional studies on frail participants suggested similar promising effects of dietary factors on frailty improvement. Nevertheless, the duration in most of the interventional studies was too short (from a few weeks to 6 months) to yield long-term outcomes (10, 12). Therefore, the effect of dietary intake on frail transitions is unknown.
In this retrospective study, we focused on prefrail individuals at baseline and examined changes in their status at the 2-year follow-up. We aimed to evaluate the association between baseline dietary intake and 2-year frailty transitions to clarify the dietary/nutritional factors that are related to frailty deterioration and reversal among older community dwellers.
Methods
Study design and participants
Data for this survey were obtained from the National Institute for Longevity Sciences-Longitudinal Study of Aging (NILS-LSA), which used detailed questionnaires, medical checkups, anthropometric measurements, physical fitness tests, and nutritional examinations to assess the normal aging process over time. NILS-LSA participants comprised randomly selected age- and sex-stratified individuals from a pool of community-dwelling residents of Obu City and Higashiura Town, which are in the neighborhood of the National Center for Geriatrics and Gerontology in Aichi Prefecture, Japan. The initial survey of the NILS-LSA involved 2,267 men and women aged between 40 and 79 years, including approximately 280 men and 280 women in each decade of age. These subjects were followed-up every 2 years from the first wave (November 1997–April 2000) to the second wave (April 2000–May 2002), third wave (May 2002–May 2004), fourth wave (June 2004–July 2006), fifth wave (July 2006–July 2008), sixth wave (July 2008–July 2010), and seventh wave (July 2010–July 2012). When participants could not be followed up (e.g., moved to another area, dropped out for personal reasons, or died), except for those aged 80 years or older, new decade- and sex-matched participants were randomly recruited from the second to seventh waves. Moreover, participants aged ≥40 years were newly recruited every year. Each wave included approximately 2300 men and women. The Committee on the Ethics of Human Research of the National Center for Geriatrics and Gerontology approved the study protocol (No. 899-6), and written informed consent was obtained from all participants. Details of the NILS-LSA have been reported previously (13).
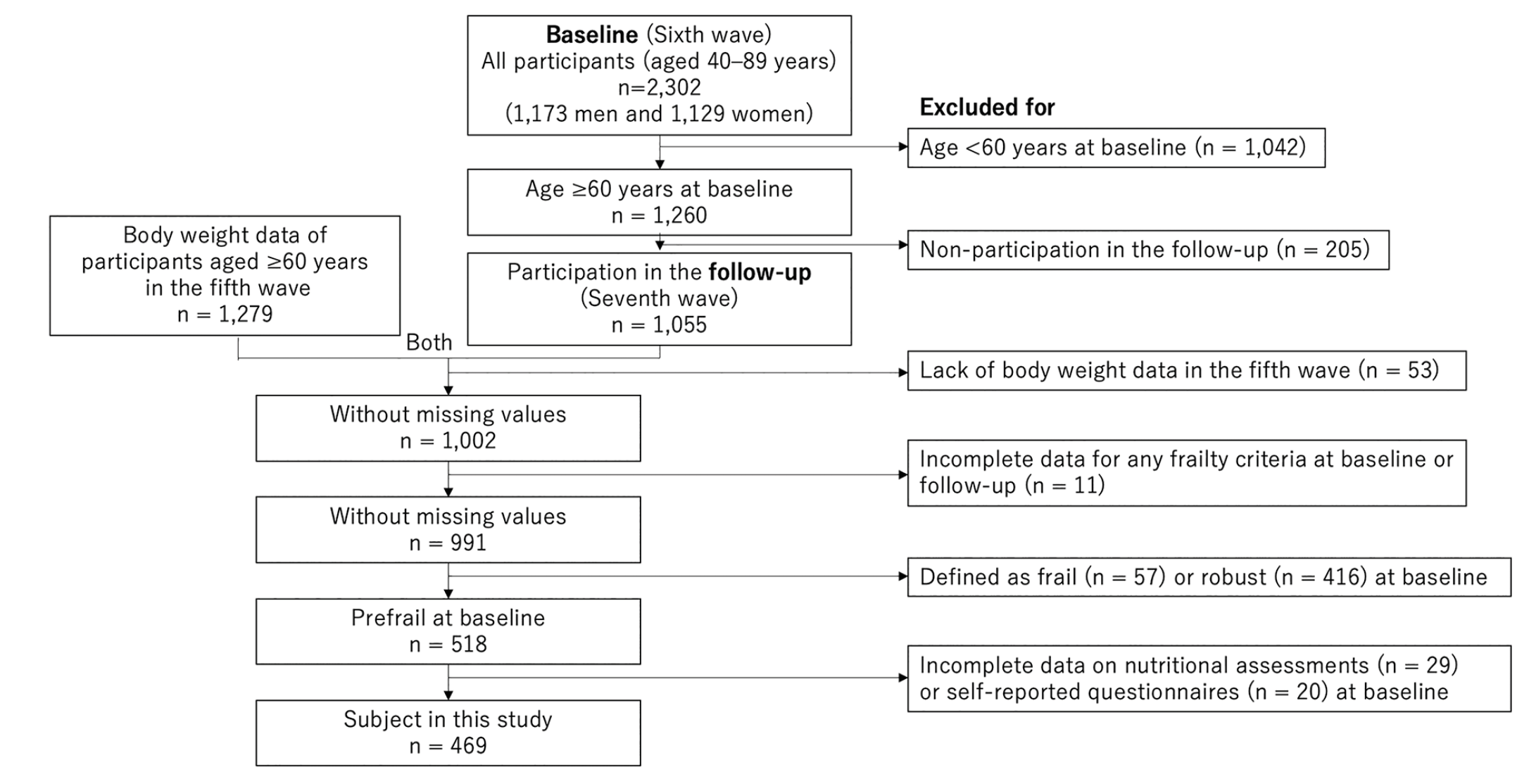
Given the small number of older adults in the first to fifth waves of the NILS-LSA, the sixth wave was used as the baseline, and the seventh wave was used as the follow-up survey (Figure 1). The main study of NILS-LSA sixth wave included participants aged 40 to 89 years, but this study focused on older adults aged ≥60 years. We considered individuals aged ≥60 years as older adults.
The baseline included 1,173 men and 1,129 women (age range: 40–89 years), and the exclusion criteria (shown in Figure 1) were as follows: 1) age <60 years at baseline (n = 1,042), 2) non-participation in the follow-up (n = 205), 3) lack of body weight measurement at the fifth wave (n = 53; this was important because shrinking at baseline could not be assessed otherwise), 4) incomplete data of any frailty criterion at baseline (n = 11), 5) definition as frail (n = 57) or robust (n = 416) at baseline, and 6) incomplete data on nutritional assessments (n = 29) or self-reported questionnaires (n = 20) at baseline.
Ultimately, the data of 469 Japanese older adults (232 men and 237 women, aged 60–87 years) were available for analysis. The mean (standard deviation) interval between baseline and follow-up for all participants was 2.0 (0.1) years.
Frailty assessment and transitions
Frailty was assessed using five criteria—slowness, weakness, exhaustion, low activity, and shrinking—based on a modified set of criteria established by the Cardiovascular Health Study (14). Details of the modified criteria have been reported previously (15).
In brief, slowness was defined as gait disturbance or a gait speed <1.0 m/s in a 10-m walk test using a comfortable gait. Weakness was defined as a maximum grip strength <26 and <18 kg for men and women, respectively. Exhaustion was assessed through self-reports based on responses to two statements in the Center for Epidemiologic Studies Depression Scale (16): the statements included “I felt that everything I did was an effort,” and “I could not get ‘going.’” The responses included “Rarely or none of the time (less than 1 day during the past week),” “Some or a little of the time (1–2 days),” “Occasionally or a moderate amount of time (3–4 days),” and “Most or all of the time (5–7 days).” Participants who did not answer “Rarely or none of the time” for both statements were identified as showing exhaustion. Low activity was defined as the lowest 20% metabolic equivalents of leisure-time physical activity based on sex, assessed using the modified Minnesota Leisure-time Physical Activity Questionnaire (17). Shrinking was defined as a ≥5% weight loss within the preceding 2 years (during the fifth to sixth [baseline] and sixth to seventh [follow-up] waves).
Frailty was defined as meeting 3 or more criteria, prefrailty was defined as meeting 1–2 criteria, and robustness was defined as not meeting any of the prespecified frailty criteria. Thus, transitions of frailty among prefrail participants at baseline (n = 469) were categorized into three groups according to changes in status from baseline to follow-up: “deterioration (prefrail to frail),” “persistence (persistent prefrail),” and “reversal (prefrail to robust).”
Supplemental Table 1 shows the prevalence of each frailty criterion at baseline and follow-up according to frailty transitions.
Nutritional assessments
On the day they participated in the baseline survey, trained dietitian staff explained the purpose and methods of the dietary record survey during lunchtime. The participants were instructed to take the survey on days when they ate normal meals as much as possible, avoiding special days such as anniversaries, because the dietary survey assessed their normal dietary habits. During this time, a 1-kg kitchen scale (Sekisui Jushi, Tokyo, Japan) and one and/or more disposable camera (27 shots; Fuji Film, Tokyo, Japan) were provided to all participants, and a practice session on taking pictures with the disposable camera was also conducted (Appendix Figure 1).
After participation in the baseline study, subjects completed a 3-day dietary record to assess dietary intake, including supplement use. The dietary record was completed over 3 consecutive days (2 weekdays and 1 day in the weekend) since food habits differed on weekdays and weekends; 3 consecutive days were selected based on the same method used by the National Health and Nutrition Examination Survey in Japan (18, 19).
All food items, including spices and seasonings, were weighed/measured separately on the 1-kg kitchen scale with a lightweight cup or spoon before being cooked, or the portion sizes were estimated. During the 3 days, all activities of eating and drinking, including consuming snacks, were noted in detail. In addition, subjects used the disposable camera to take photos of their meals before and after eating. When taking the photos, they were asked to place a scale paper on the dining table so that we could estimate the size of the plates and foods. If it was difficult for the subjects to write detailed records (i.e., if they were not in charge of cooking), the cooks conducted the recording. Subjects completed the dietary record at home, and most returned it within 1 month.
The films in the returned disposable cameras were developed. Dietitians then used these photos to complete the missing information in each subject’s dietary record and assigned a code number from the Standard Tables of Food Composition in Japan 2010 (20) for every food (the number of codes in each subject’s records was around a few hundreds). Based on the code number and weight records, we used the Statistical Analysis System software version 9.3 (SAS Institute, Cary, NC, USA) to calculate the average food and nutrient intake (including alcohol intake) over 3 days from the nutrient intakes included in the Standard Tables of Food Composition in Japan 2010 (20), without using any dietary assessment software. There are 17 food groups in the Japanese food composition table (14): cereals, potatoes, sugars and sweeteners, beans, nuts and seeds, vegetables (non-green-yellow/green-yellow), fruits, mushrooms, seaweed, fish and shellfish, meats, eggs, milk and dairy products, fats and oils, confectionaries, beverages, and seasoning and spices. In this study, we included 13 food groups: cereals, potatoes, beans, nuts and seeds, non-green-yellow vegetables, green-yellow vegetables, fruits, mushrooms, seaweed, fish and shellfish, meats, eggs, milk and dairy products, and excluded sugars and sweeteners, fats and oils, confectionaries, beverages, and seasoning and spices. Each food group contains dozens or hundreds of foods, for example, the milk and dairy products group contains the nutritional values of 52 different foods, including natural milk, low-fat milk, yogurt, cheese, and butter.
Calculating the nutrient values for each subject based on the coding chart took several hours even for a trained dietitian, and the values were checked by two or more other trained dietitians to prevent coding errors. Information on any discrepancies and any requisite additional information was obtained via telephone calls to the subjects. However, if the records were still insufficient, the dietary survey data were considered missing.
Other measurements
Weight and height were measured in the fasting state (around 9–10 am) to the nearest 0.1 kg and 0.1 cm, respectively, with participants wearing light clothing and no shoes. The body mass index was calculated as the body weight in kilograms divided by the square of the height in meters. Data on years of education (≤12 or ≥13 years of school), annual family income (<5.5 or ≥5.5 million yen per year), smoking status (current, never/former), and chronic disease history (past and present stroke, heart disease, hypertension, dyslipidemia, and diabetes mellitus; yes/no, for each) were collected using a self-administered questionnaire and confirmed by medical doctors or trained staff.
Statistical analysis
According to frailty transitions, differences in proportions and means of baseline characteristics were assessed using the chi-squared test and general linear model, respectively. As for dietary variables, we selected the parametric test (general linear model) because each variable is a continuous quantity and the distribution was close to a normal distribution. A general linear model adjusted for sex, baseline age, years of education, annual family income, smoking status, and history of chronic disease was applied to compare baseline nutrient intakes according to the frailty transitions. Moreover, to compare baseline food intakes according to frailty transitions, general linear regression was performed again. Model 1 was adjusted for sex, baseline age, years of education, annual family income, smoking status, and chronic disease history. Model 2 was further adjusted for the total number of frailty criteria at baseline. BMI and physical activity were not treated as an adjustment factor because 1) the weight used in the BMI calculation was used to determine the weight loss (shrinking) of the frailty component, 2) the leisure-time physical activity was used to determine the low activity of the frailty component.
To test any trend in the association between dietary intakes and frailty transitions, the trend test of the general linear model was applied with ascending ordinal values −1, 0, and 1 assigned to “deterioration (prefrail to frail),” “persistence (persistent prefrail),” and “reversal (prefrail to robust),” respectively. In sub-analyses, we focused on dairy intakes and estimated the daily mean dairy intakes (including milk, yogurt, and cheese) according to frailty transitions using the general linear model. Adjustments were the same as those in Model 2.
All statistical analyses were performed using the Statistical Analysis System software version 9.3 (SAS Institute, Cary, NC, USA). All reported P-values were two-sided, and a P-value <0.05 was considered significant.
Results
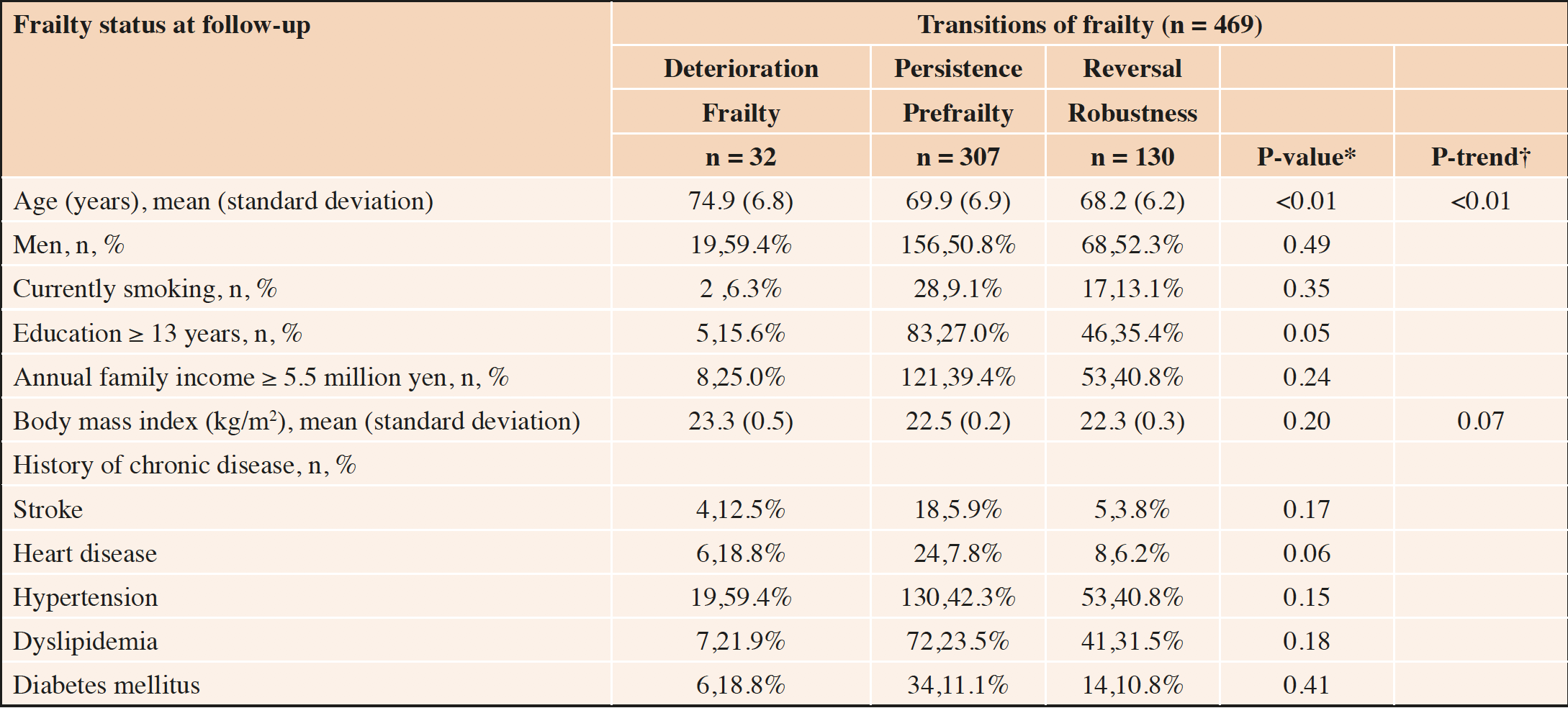
At the 2-year follow-up, 28% (n = 130) of participants had reversed to robust status, whereas 7% (n = 32) and 65% (n = 307) had deteriorated to frail and remained at prefrail statuses, respectively. Participants in the reversal group were younger than those in the other two groups. Although the prevalence of chronic disease (except dyslipidemia) tended to be high in the deterioration group, no significant differences were observed (Table 1).
*General linear model used for continuous variables; χ2 test used for the categorical variables; †Trend test of the general linear model used with ascending ordinal values −1, 0, and 1 assigned to “deterioration,” “persistence,” and “reversal,” respectively.
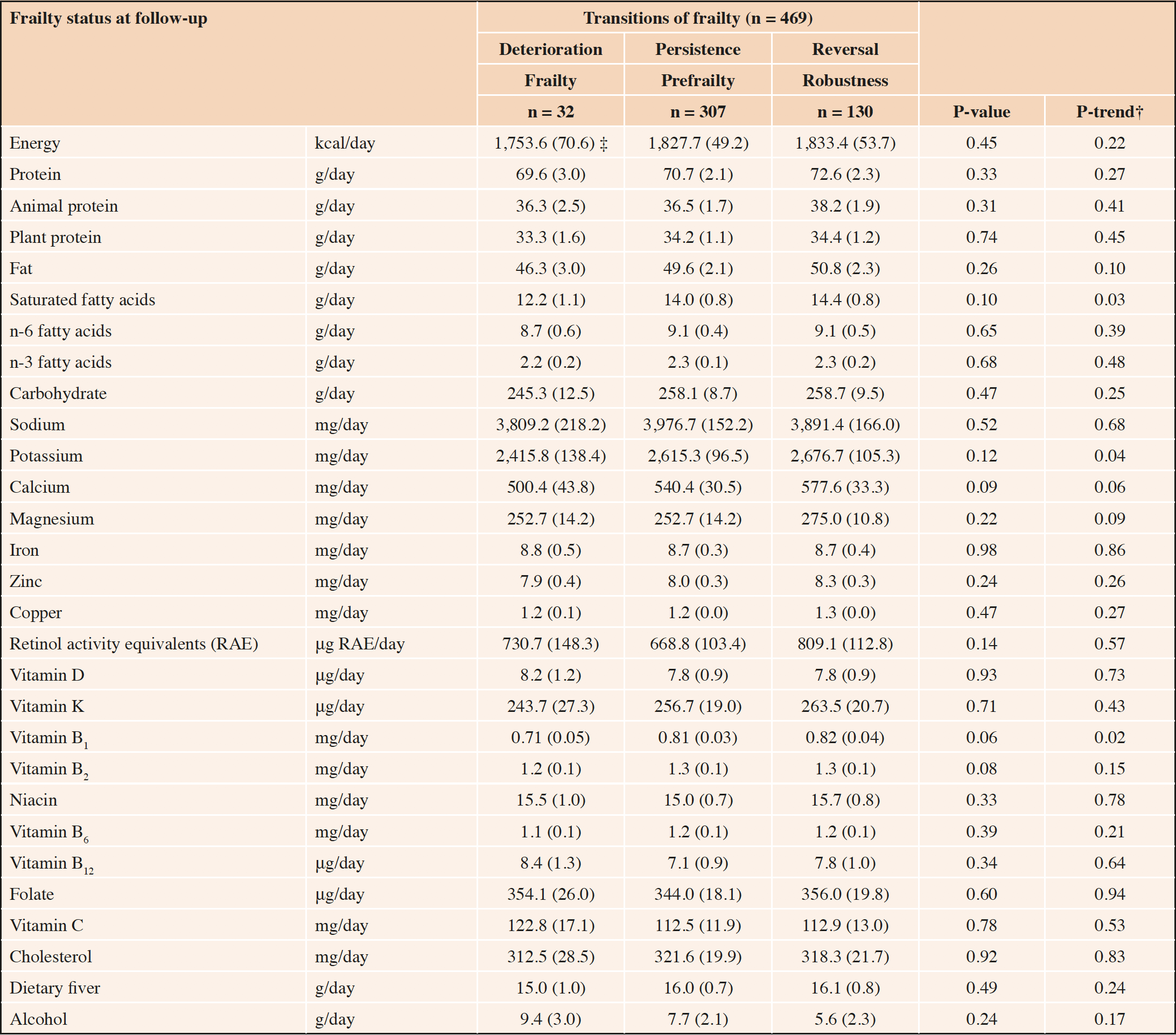
Table 2 shows the multivariate-adjusted baseline nutrient intakes according to transitions of frailty. After adjusting for sex, baseline age, years of education, annual family income, smoking status, and chronic disease history, the baseline intake of saturated fatty acids, potassium, and vitamin B1 tended to be high in the reversal group (P-trend: 0.03, 0.04, and 0.02, respectively), although the differences in estimated mean intakes were not significant among the three groups.
*General linear model used. Adjusted for sex, baseline age, years of education, annual family income, smoking status, and chronic disease history; †Trend test of the general linear model used with ascending ordinal values −1, 0, and 1 assigned to “deterioration,” “persistence,” and “reversal,” respectively; ‡Estimated mean (standard error), all such values.
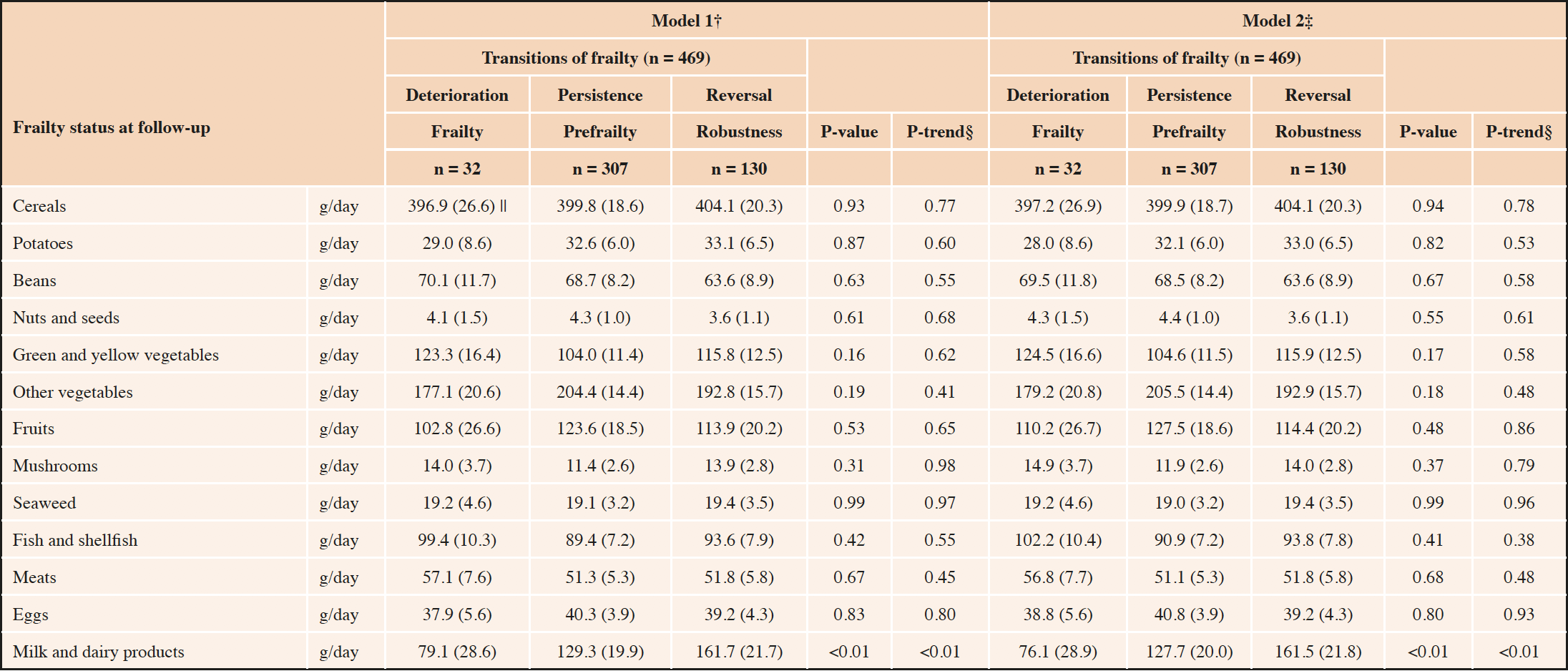
Table 3 shows the baseline food intakes according to the transitions of frailty. After multivariate adjustment, milk and dairy product intake were positively associated with frailty reversal. The corresponding estimated mean (standard error) values for milk and dairy products intake (g/day) for “frailty deterioration,” “frailty persistence,” and “frailty reversal” were 79.1 (28.6), 129.3 (19.9), and 161.7 (21.7), respectively (P-value 0.0036, P-trend 0.0019; Model 1). Even after adjusting for the total number of frailty criteria at baseline, the positive association remained (P-value 0.0028, P-trend 0.0014; Model 2). No other food intake was associated with frailty reversal.
In sub-analyses (Supplemental Table 2), milk and yogurt intake, but not cheese intake, were positively associated with frailty reversal.
*General linear model was used; †Adjusted for sex, baseline age, years of education, annual family income, smoking status, and chronic disease history. ‡Adjusted for Model 1 + total number of frailty criteria at baseline; §Trend test of the general linear model used with ascending ordinal values −1, 0, 1 assigned to “deterioration,” “persistence,” and “reversal,” respectively; ||Estimated mean (standard error), for all such values.
Discussion
This retrospective study indicated that dairy product intake was positively associated with frailty reversal among older community dwellers. To our best knowledge, this is the first study to investigate the association between dietary intake and frailty transitions.
Cuesta-Triana et al. (21) reviewed the effect of milk and other dairy products on the risk of frailty, sarcopenia, and cognitive performance decline in older adults. They concluded that dairy product consumption might reduce the risk of frailty, especially high consumption of low-fat milk and yogurt. In our study, milk and yogurt intake, but not low-fat milk or cheese intake, were associated with frailty reversal. This may be because our participants consumed relatively low amounts of low-fat dairy products (mean 21.1 g/day) and cheese (mean 2.5 g/day); therefore, the association between the intake of these products and frailty transitions was difficult to determine. Although the mean value of dairy product intake in our study was smaller than reports from previous (Spain) studies (306 g vs. 141 g per day) among robust community-dwelling older adults (21, 22), both studies observed benefits of high dairy product intake on frailty prevention. A recent study indicated that dairy consumption was associated with a lower risk of mortality and major cardiovascular disease events in a diverse multinational cohort in 21 countries (23). This suggests that daily dairy product consumption may protect frailty development both in robust and prefrail older adults.
Dairy products are good sources of protein, vitamins, and minerals (24), and these nutrients are considered to play an important role in the prevention of frailty. Although the differences in the estimated mean intakes were not significant, the intake of saturated fatty acids, potassium, and vitamin B1 tended to be highest in the reversal group among the frailty transition groups. A higher percentage of saturated fatty acid intake was associated with both higher frailty and mortality (25), even after considering the degree of nutritional deficits. However, the mean total fatty acid and saturated fatty acid intakes were higher in that study (25) compared to our sample (total fatty acid: 75.65 g vs. 46.3–50.8 g per day, saturated fatty acid: 24.77 g vs. 12.2–14.4 g per day; Table 2). Therefore, while excessive intake of saturated fatty acids is detrimental to frailty, the moderate intake of saturated fatty acids among older adults who consume small amounts of saturated fatty acid may be effective in preventing frailty.
Potassium exerts beneficial effects on cardiovascular diseases (26). The salt intake of the Japanese people is extremely high compared to the rest of the world, and in our sample, the sodium intake was 3809.2, 3976.7, and 3891.4 mg, the potassium intake was 2415.8, 2615.3, and 2676.7 mg, and the sodium/potassium ratio was 1.58, 1.52, and 1.54 for the deterioration, persistence, and reversal groups, respectively. Therefore, in a population that has a high salt intake, the high potassium intake may partly modify the frailty status through a reduction in the risk of cardiovascular disease because high sodium intake is a strong risk factor for cardiovascular disease (27). The results of this study indicate frailty reversal in older adults with high potassium intake. Among Japanese cohorts, vitamin B1, potassium, and other micronutrient intake were cross-sectionally lower in the prefrailty group (26, 28). These nutrients, including saturated fatty acids, potassium, and vitamin B1, are found abundantly in vegetables, fruits, meat, and/or fish; thus, dairy product intake may not be solely responsible for the improved nutritional status. As the nutritional status of our subjects was relatively good compared to the results of the National Health and Nutrition Examination Survey in Japan (29), the difference in the dairy intake may have affected their prognosis in terms of the frailty status.
Furthermore, the differences in the estimated mean total protein intakes were not significant among the transitions of frailty groups. Therefore, health awareness and accessibility to dairy products may play a role in improving prefrail status by promoting physical and mental health rather than simply relying on protein intake. In our previous study, the intake of dairy products and fruits decreased with age (30). These foods need to be fresh and are difficult for Japanese families and communities to procure and grow at home. Furthermore, these foods are usually heavy and difficult to carry home from the store, which may cause prefrail older adults to refrain from purchasing them. Therefore, our findings indicate that health awareness and actions may be key factors for frailty reversal.
The proportion of transitions among our prefrail participants was similar to that of a previous study. Corresponding proportions for “reversal,” “persistence,” and “deterioration” were 35%, 55%, and 7% over 1.6 years (31) (28%, 65%, and 7% over 2 years in our study). Considering that there are many prefrail older adults in the community and that frailty reversal is highly achievable, advocating dairy intake may be an important nutritional strategy to promote health and prevent frailty.
The strengths of our study are as follows: (1) this was the first study that investigated the association between dietary intake and frailty transitions among prefrail older participants; (2) dietary and nutritional intake was assessed by 3-day dietary records and photographs, which may introduce less recall bias than other methods (e.g., dietary recall and self-reported questionnaires); (3) the estimated mean consumptions of milk and yogurt were 96.5 g/day and 36.4 g/day in the “frailty reversal” group, which are almost half a cup of milk and yogurt. Therefore, if accessed sustainably, even older adults could easily consume these volumes. In other words, at the level of public health policy formulation and advocacy, our findings provide more feasible evidence for frailty prevention and health promotion.
Our study has a few limitations that warrant consideration. First, dietary and nutritional intake was only assessed at baseline. Diet naturally changes over time, and it is affected by various factors related to aging. Second, a 3-day dietary record was not enough to accurately assess long-term eating habits. In addition, the Japanese intake of dairy products is relatively lower than the average global intake (29, 32). Therefore, our findings may not be generalizable to Western populations who consume larger amounts of dairy products. However, even among Western populations, the consumption of dairy products would decline with age; thus, our findings may be valuable for those populations.
In conclusion, consumption of dairy products, including milk and yogurt, might be beneficial for promoting frailty reversal and frailty prevention among older community dwellers who consume small amounts of dairy products. Our findings might be applicable for robust and frail individuals. Future studies are needed to address this hypothesis.
Funding: This work was supported in part by grants from the Food Science Institute Foundation and Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology, Japan (grant number 19-10,21-18). The sponsors had no role in the design and conduct of the study; in the data collection, analysis, and interpretation; in the preparation of the manuscript; or in the review or approval of the manuscript.
Acknowledgments: We wish to express our sincere appreciation to the study participants and our colleagues in the NILS-LSA for completing the surveys in this study.
Conflicts of interest: All authors declare no conflicts of interest.
Ethical Standards: This study was carried out in accordance with the ethical standards.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019;394:1365-1375. doi: 10.1016/S0140-6736(19)31786-6.
2. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009.
3. Fallah N, Mitnitski A, Searle SD, Gahbauer EA, Gill TM, Rockwood K. Transitions in frailty status in older adults in relation to mobility: a multistate modeling approach employing a deficit count. J Am Geriatr Soc 2011;59:524-529. doi: 10.1111/j.1532-5415.2011.03300.x.
4. Michel JP, Cruz-Jentoft AJ, Cederholm T. Frailty, Exercise and Nutrition. Clin Geriatr Med 2015;31:375-387. doi: 10.1016/j.cger.2015.04.006.
5. Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res Rev 2019;50:81-88. doi: 10.1016/j.arr.2019.01.010.
6. Gill TM, Gahbauer EA, Allore H, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418-423. doi: 10.1001/archinte.166.4.418.
7. Mendonça N, Kingston A, Yadegarfar M, et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing 2020;49:974-981. doi: 10.1093/ageing/afaa054.
8. Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018;3:e323-e332. doi: 10.1016/S2468-2667(18)30091-4.
9. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open 2018;8:e018195. doi: 10.1136/bmjopen-2017-018195.
10. Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism 2017;68:64-76. doi: 10.1016/j.metabol.2016.12.005.
11. Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr 2017;17:108. doi: 10.1186/s12877-017-0496-2.
12. Morante JJH, Martínez CG, Morillas-Ruiz JM. Dietary factors associated with frailty in old adults: a review of nutritional interventions to prevent frailty development. Nutrients 2019;11:102. doi: 10.3390/nu11010102.
13. Shimokata H, Ando F, Niino N. A new comprehensive study on aging–the National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS-LSA). J Epidemiol 2000;10:S1-S9. doi: 10.2188/jea.10.1sup_1.
14. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146.
15. Yuki A, Otsuka R, Tange C, et al. Epidemiology of frailty in elderly Japanese. J Sports Med Phys Fitness 2016;5:301-307.
16. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401.
17. Kozakai R, Doyo W, Tsuzuku S, et al. Relationships of muscle strength and power with leisure-time physical activity and adolescent exercise in middle-aged and elderly Japanese women. Geriatr Gerontol Int 2005;5:182-188.
18. Imai T, Sakai S, Mori K, Ando F, Niino N, Shimokata H. Nutritional assessments of 3-day dietary records in National Institute for Longevity Sciences–Longitudinal Study of Aging (NILS-LSA). J Epidemiol 2000;10:S70-S76. doi: 10.2188/jea.10.1sup_70.
19. Yoshiike N, Matsumura Y, Iwaya M, Sugiyama M, Yamaguchi M. National Nutrition Survey in Japan. J Epidemiol 1996;6:S189-200. doi: 10.2188/jea.6.3sup_189.
20. Ministry of Education Culture, Sports, Science and Technology. Standard Tables of Foods Composition in Japan 2010. Ishiyaku-syuppan.
21. Cuesta-Triana F, Verdejo-Bravo C, Fernández-Pérez C, Martín-Sánchez FJ. Effect of milk and other dairy products on the risk of frailty, sarcopenia, and cognitive performance decline in the elderly: a systematic review. Adv Nutr 2019;10:S105-S119. doi: 10.1093/advances/nmy105.
22. Lana A, Rodriguez-Artalejo F, Lopez-Garcia E. Dairy consumption and risk of frailty in older adults: a prospective cohort study. J Am Geriatr Soc 2015;63:1852-1860. doi: 10.1111/jgs.13626. doi: 10.1111/jgs.13626.
23. Dehghan M, Mente A, Rangarajan S, et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018;392:2288-2297. doi: 10.1016/S0140-6736(18)31812-9.
24. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res 2016;60:32527. doi: 10.3402/fnr.v60.32527.
25. Jayanama K, Theou O, Godin J, Cahill L, Rockwood K. Association of fatty acid consumption with frailty and mortality among middle-aged and older adults. Nutrition 2020;70:110610. doi: 10.1016/j.nut.2019.110610.
26. Liu F, Zhang R, Zhang W, et al. Potassium supplementation blunts the effects of high salt intake on serum retinol-binding protein 4 levels in healthy individuals. J Diabetes Investig 2021;12:658-663. doi: 10.1111/jdi.13376.
27. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014;129:981-989. doi: 10.1161/CIRCULATIONAHA.
28. Kaimoto K, Yamashita M, Suzuki T, et al. Association of protein and magnesium intake with prevalence of prefrailty and frailty in community-dwelling older Japanese women. J Nutr Sci Vitaminol (Tokyo) 2021;67:39-47. doi: 10.3177/jnsv.67.39.
29. Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan. 2009. http://www.mhlw.go.jp/bunya/kenkou/eiyou/h21-houkoku.html. Accessed [17 April 2021].
30. Otsuka R, Nishita Y, Tange C, et al. Dietary diversity decreases the risk of cognitive decline among Japanese older adults. Geriatr Gerontol Int 2017;17:937-944. doi: 10.1111/ggi.12817.
31. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418-423. doi: 10.1001/archinte.166.4.418.
32. Micha R, Khatibzadeh S, Shi P, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ 2014;348:g2272. doi: 10.1136/bmj.g2272.