L. Swan1, A. Warters2, M. O’Sullivan1
1. Department of Clinical Medicine, School of Medicine, Trinity College Dublin, Dublin, Ireland; 2. Older Person Services, Dublin North City and County Community Health Organisation, Health Service Executive, Dublin, Ireland
Corresponding Author: Lauren Swan, Department of Clinical Medicine, School of Medicine, Trinity College Dublin, Dublin, Ireland E: swanla@tcd.ie, T: +353 857 824 606
J Frailty Aging 2022;11(4)398-406
Published online May 2, 2022, http://dx.doi.org/10.14283/jfa.2022.32
Abstract
Background: Sarcopenia is characterized by the accelerated loss of muscle strength, mass, and function in aging. The disease is a major public health issue with emerging evidence of a disproportionate burden in areas of socioeconomic disadvantage.
Objectives: To estimate the prevalence of probable sarcopenia overall, and according to Socioeconomic Position (SEP). To explore the association between markers of SEP and probable sarcopenia.
Design: Cross-sectional analysis of the English Longitudinal Study of Ageing data.
Setting: England, United Kingdom (UK).
Participants: This study comprised 6,052 older adult participants from Wave 6 of the English Longitudinal Study of Ageing (ELSA) aged 60 years and older.
Measurements: Probable sarcopenia was identified by the EWGSOP2 guidelines as low hand grip strength (females <16kg and males <27kg) or poor chair rise test performance (completion of 5 chair rises >15 seconds). Socioeconomic position was defined by educational attainment and subjective social status (SSS). Weighted multivariable regression analysis was employed to identify determinants of probable sarcopenia.
Results: Over one-third of older adults met the criteria for probable sarcopenia (33.7%; weighted, 36.1%) in the study population of mean age 70.7 (SD 7.7) years. When examined by SEP, the prevalence of probable sarcopenia was over 2-fold higher in adults in the most vs the least disadvantaged SEP groups (47.0% vs 20.6%, respectively, p<0.001). Multivariable regression analysis identified disadvantaged SEP, as measured by educational attainment and SSS, as independent predictors of probable sarcopenia, along with older age, physical inactivity, underweight BMI, chronic conditions, osteoarthritis, and minority group ethnicity.
Conclusions: Disadvantaged SEP was associated with an increased likelihood of probable sarcopenia when controlled for other known risk factors. The findings suggest a need and opportunity for sarcopenia prevention and treatment strategies to address socioeconomic disadvantage in policies and practice.
Key words: Sarcopenia, socioeconomic position, aging, health inequality.
Introduction
There is growing recognition of the impact of social determinants on health across the life course (1, 2). Socioeconomic disadvantage is associated with accelerated aging, multimorbidity, poorer health outcomes and reduced access to healthcare (3–6). Recent findings suggest associations between socioeconomic disadvantage and sarcopenia, (7–9) however, there is a paucity of research in this area.
Sarcopenia is a muscle disease characterized by the accelerated loss of muscle strength, mass and function (10). This disease is a major public health issue and contributor to limitations in activities of daily living, functional decline, frailty, disability, hospitalization and mortality amongst older populations (11–13). In 2018, the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) introduced the concept of ‘probable sarcopenia’ defined by weak muscle strength (10). This emphasis on muscle strength as the principal determinant of sarcopenia recognizes that low strength, rather than mass, is a robust predictor of adverse health outcomes in older adults (14–16). Sarcopenia is probable when upper or lower limb muscle strength is deemed low, often categorized by hand grip strength less than 16 kg in females or 27 kg in males or completion of 5 chair rises in a time greater than 15 seconds (10). Probable sarcopenia can readily be measured in large populations with simple, cost-effective approaches and is the focus of the present study. Importantly, the detection of probable sarcopenia is considered sufficient evidence to initiate appropriate interventions (10).
Probable sarcopenia affects an estimated 5-73% of community-dwelling older adults, with the highest occurrence observed amongst cohorts of advanced age (85+ years old) (8, 17–20). Arguably, variation in prevalence estimates may be due to mode of assessment of probable sarcopenia (18). While recent studies identify links between socioeconomic disadvantage and low hand grip strength or lower extremity strength in later life (6, 9), few have examined associations using EWGSOP2 guidelines for probable sarcopenia. Older age, physical inactivity and multimorbidity are documented risk factors for probable sarcopenia, though few studies have examined socioeconomic factors (17). The present study uses the term socioeconomic position (SEP), a concept applied in health research, which refers to the economic and social factors that affect an individual’s position within society (21, 22). SEP is commonly operationalized as income, wealth, occupational class, subjective social status (SSS) and educational attainment (6, 23, 24). The present study uses indicators of SEP which may reflect different time points across the life course. Educational attainment may represent SEP in childhood and into adulthood, SSS was self-reported at the time of the study representing participants’ current perception of SEP as older adults (25). Socioeconomic disadvantage shows geographic variations, and we explored if sarcopenia prevalence may mirror the identified regional patterns for health inequality reported in England (26, 27).
Findings on SEP and sarcopenia are emerging however, the results remain inconsistent. Brennan-Olsen et al, in a 6-year longitudinal analysis of older adults in Australia, reported lower hand grip strength and lower extremity strength in disadvantaged SEP groups, classified by educational attainment and occupation class (9). Pérez-Sousa et al, in an ethnically diverse study from Columbia, reported that individuals in the lowest socioeconomic status groups, defined by social class, had an increased risk of probable sarcopenia when compared to the high SEP group (8). Dodds et al included a measure of occupation class in a large study of UK older adults, but an association with probable sarcopenia was not apparent when controlled for other known risk factors (18). Recently, we showed that socioeconomic disadvantage, defined by educational attainment, was an independent predictor of probable sarcopenia in a large population of Irish older adults (n= 3342) (7). There remains a gap in the literature for studies specifically focusing on sarcopenia risk and SEP and in populations with greater ethnic diversity. A better understanding of sarcopenia and socioeconomic disadvantage may be useful in the provision of screening, interventions, and resource allocation for those most at risk of sarcopenia.
The present study aimed to describe the prevalence of probable sarcopenia, according to EWGSOP2 guidelines, overall and according to SEP and mapped to geographic area of residence in England. We further explored SEP, along with known variables, as determinants of probable sarcopenia. We hypothesized that probable sarcopenia would be more prevalent in older adults with disadvantaged compared with higher SEP, defined by educational attainment and subjective social status.
Methods
Study Design and Population
This study was a cross-sectional analysis of data from Wave 6 of the English Longitudinal Study of Aging (ELSA), which is an ongoing nationally representative longitudinal study of older adults in England. Wave 6 was conducted in 2012-2013 and included 10,601 community-dwelling adults aged 50 years and over, recruited from the Health Survey of England cohort. Of these, 8,054 participants enrolled in a nurse-led health assessment. Further details regarding the study design have been previously reported (28).
For the present study, the inclusion criteria were as follows: adults living in private households, aged 60 years and older, participation in the study health assessment and available data on hand grip strength or chair rise test performance (Figure 1). Written informed consent was obtained from all participants and ethical approval was granted from the National Research Ethics Service (MREC/01/2/91).
Assessing Probable Sarcopenia
Probable sarcopenia was defined according to the European Working Group on Sarcopenia in Older People (EWGSOP2) guidelines for weak hand grip strength or poor chair-rise test performance (10). Grip strength was assessed using a Smedley dynamometer (Stoelting Co, IL, USA) with three measurements taken for each hand and the maximum value for the dominant hand used in analyses, similar to previous studies (29). We applied the EWGSOP2 gender-specific cut-off values for low hand grip strength defined as less than 16 kg in females or 27 kg in males (10).
Chair rise test performance, an indicator of lower body strength, was recorded as the time taken by a participant to stand up from a firm chair, without using their arms, 5 times. A time greater than 15 seconds to complete 5 chair rises was classified as probable sarcopenia (10). Participants unable to complete the chair rise test unassisted by arms or feeling unsafe were categorized as having poor chair rise performance (n= 677, 11.2%). Participants missing measures for hand grip strength (n= 80, 1.3%) were assessed by chair rise test performance alone. In line with EWGSOP2 guidelines, probable sarcopenia was defined by a participant meeting the criteria for low hand grip strength or poor chair rise performance (10).
Socioeconomic Position (SEP)
Educational attainment was used as a marker of SEP. Four SEP groups were derived according to the self-reported highest level of formal education completed at Wave 6. Qualifications were classified as previously described (30, 31), as Group 1) no formal educational qualifications or completion of a Level 1 National Vocational Qualification (NVQ). Group 2) secondary school lower: completed O-Levels or equivalent, a Level 2 NVQ or a non-coded internationally obtained qualification. Group 3) secondary school upper: completion of GCE A-Levels or equivalent, NVQ Level 3, or a professional qualification below degree level. Group 4) completion of a third-level degree or NVQ levels 4-5.
Subjective Social Status (SSS) was a self-reported measure of perceived position within the social hierarchy (32). Participants were asked to mark their perceived social standing on a ladder, where the highest rung represented ‘the best off, with the best education, income and jobs’ and the bottom of the ladder represented the position of the ‘worst off’. Participants were asked to mark a cross on the ladder rung which they perceived to reflect their position relative to society. Possible scores on the ladder ranged from 5 to 100, with lower scores indicating greater socioeconomic disadvantage. The ladder rung scores were classified into four groups, where Q1 represented the least disadvantaged (score 75-100) and Q4 the greatest subjective disadvantage (score 5-25) (33).
Health and Lifestyle Risk Factors
Demographic variables included sex (dichotomous variable); age (continuous variable) top-coded at 91 years old to preserve anonymity. Information on ethnicity was available as a dichotomous variable described as white or ethnic minority group. We used the area of residence within England to describe the geographical spread of probable sarcopenia; this was categorized using Government Office Region (GOR).
Health and lifestyle risk factors for probable sarcopenia were selected based on current published evidence. Self-reported physical activity data were collected, with participants asked about the frequency of vigorous, moderate, and mild intensity physical activities. Participants were shown prompt cards illustrating examples of activities associated with each level of physical intensity. Subjective physical activity was classified into vigorous, moderate, and low levels. Self-reporting vigorous activity at least once per week was classified as high physical activity. Moderate physical activity was indicated by no vigorous activity, but moderate activity at least once per week. Low physical activity was present if the frequency of vigorous or moderate activity was lower than once per week (34). Chronic conditions were classified according to the modified Functional Comorbidity Index (FCI) which produced a continuous score (0-14) of long-term conditions (35). Self-reported smoking status was recorded as current smoker, past smoker or never smoker. Osteoarthritis was documented as present if a diagnosis was confirmed from previous waves, or if newly reported in Wave 6.
Anthropometric measurements, collected during the nurse health visit, included Body Mass Index (BMI) and Waist Circumference (WC). Body Mass Index (BMI), calculated by weight (kg) and height (m2) was classified according to standard criteria: underweight (≤18.5 kg/m2), normal (>18.5-25 kg/m2), overweight (>25-30 kg/m2) and obese
(>30 kg/m2) (35). Waist circumference was classified based on gender-specific cut-off values for females: normal fat distribution (<80 cm), moderate central fat accumulation (80-87.9 cm) and high central fat accumulation (>88 cm) and males: normal fat distribution (<94 cm), moderate central fat accumulation (94-101.9 cm) and high central fat accumulation (>102 cm) (36).
Statistical Analysis
Descriptive statistics are reported as means ± SD or percentages. Normality was assessed for all variables by Shapiro-Wilks tests. Cross-sectional weights were applied to the study population to minimize bias arising from differences in participation rates in the nurse visit. The Wave 6 cross-sectional weight was modelled on age, sex, region, housing tenure, educational qualifications, and marital status to bring the study population in line with 2012 population estimates for England. Similarly, the nurse-visit weighting strategy was calibrated for 2012 population estimates in England across the previously described sociodemographic indices. The nurse-visit final weight was a combination of the estimated probability of nurse-visit response and the wave 6 cross-sectional weight. Further details on the nurse-visit weighting strategy applied are described elsewhere (37). The final nurse-visit weight was applied to the multivariable logistic regression.
Chi-square test for independence was applied to compare demographic, health and lifestyle variables of interest between the probable sarcopenic and reference groups. Continuous variables were compared between groups using Independent Student t-tests. Pre-selected risk factors of probable sarcopenia, low hand grip strength and poor chair rise test performance, respectively, were assessed using multivariable logistic regression analysis. Multicollinearity was assessed using correlation matrices and Variance Inflation Factors (VIFs). Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are reported for multivariable analyses. All analyses were performed using IBM SPSS Statistics V24 software
Results
Study Population
At Wave 6, nurse-led health assessments were conducted with 8,054 participants (Figure 1). Of these, 6,052 were aged 60 years and older with measured hand grip strength or chair rise test performance. Thus, the analytical sample comprised 6052 community-dwelling older adults of mean age (SD) 70.7 (7.7) years, 54.4% were female and 14.5% were aged 80 years or older. Most were of white ethnicity (97.7%) (Table 1). Based on education as a marker of SEP, 27.4% completed no formal qualifications (n= 1658), 28.9% lower secondary (n= 1748), 25.6% upper secondary (n= 1550) and 18.0% a third-level degree (n= 1088). Additionally, 3.0% reported SSS in the most disadvantaged ladder rungs (n= 160) and 16.8% (n= 899) as least disadvantaged. The study population had a high frequency of overweight and obesity (70.1%), physical inactivity (74.9%) past cigarette smoking (54.5%), co-morbidity (47.5%) and osteoarthritis (30.2%).
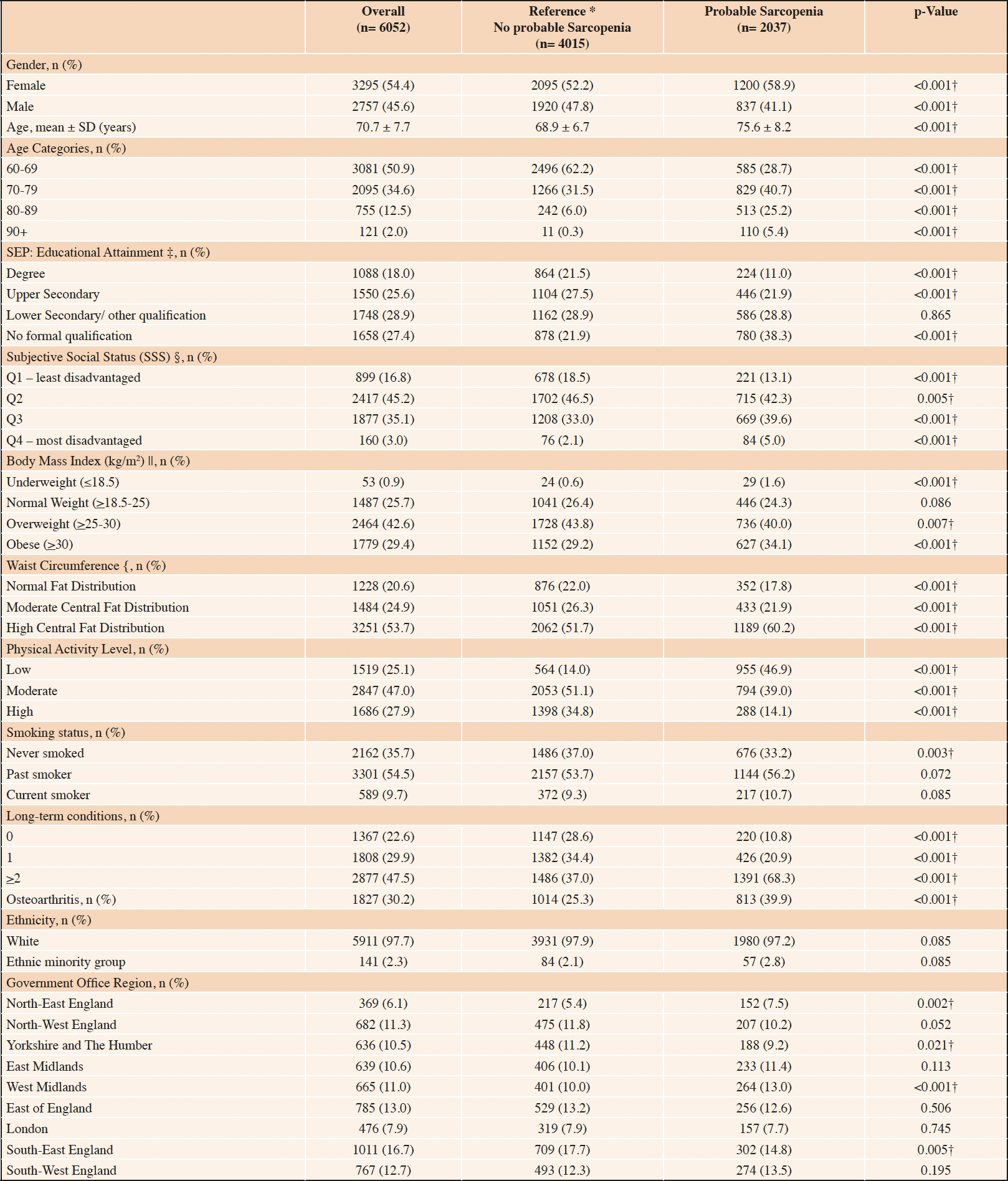
Table 1. Characteristics of the study population overall and based on the presence of probable sarcopenia as defined by EWGSOP2 criteria (n= 6052)
*Did not meet the criteria for probable sarcopenia based on the EWGSOP2 cut-offs for weak hand grip strength or poor chair rise test performance; Chi-squared χ2 and independent student t-test used for comparisons between sarcopenic and reference groups (†p <0.05); Complete case analysis; Missing data, n (%); ‡ 8 (0.1%),§ 699 (11.5%); || 269 (4.4%); { 89 (1.5%)
Prevalence of Probable Sarcopenia overall and by SEP
Probable sarcopenia was detected in 33.7% of this study population (weighted 36.1%), as defined by the EWGSOP2 guidelines (Table 1). When examined based on educational attainment, probable sarcopenia was over 2-fold higher among participants who reported no formal educational qualifications (47.0%, weighted 49.4%) compared with those with a third-level qualification (20.6%, weighted 20.3%, p<0.001) (Figure 2). The prevalence of probable sarcopenia in the intermediate SEP groups, upper secondary qualifications and lower secondary/other qualifications was (33.5%, weighted 35.2% and 28.8%, weighted 29.1%, respectively). Similarly, probable sarcopenia was highest among older adults with disadvantaged SSS (52.5%, weighted 57.3%) and lowest in older adults with greater SSS (24.6%, weighted 25.8%). When mapped to participants’ region of residence in England, probable sarcopenia was highest in the North-East of England (41.2%, weighted 43.0%) and lowest in Yorkshire and the Humber (29.6%, weighted 32.7%) and the South East of England (29.9%, weighted 31.9%) (Figure 3).
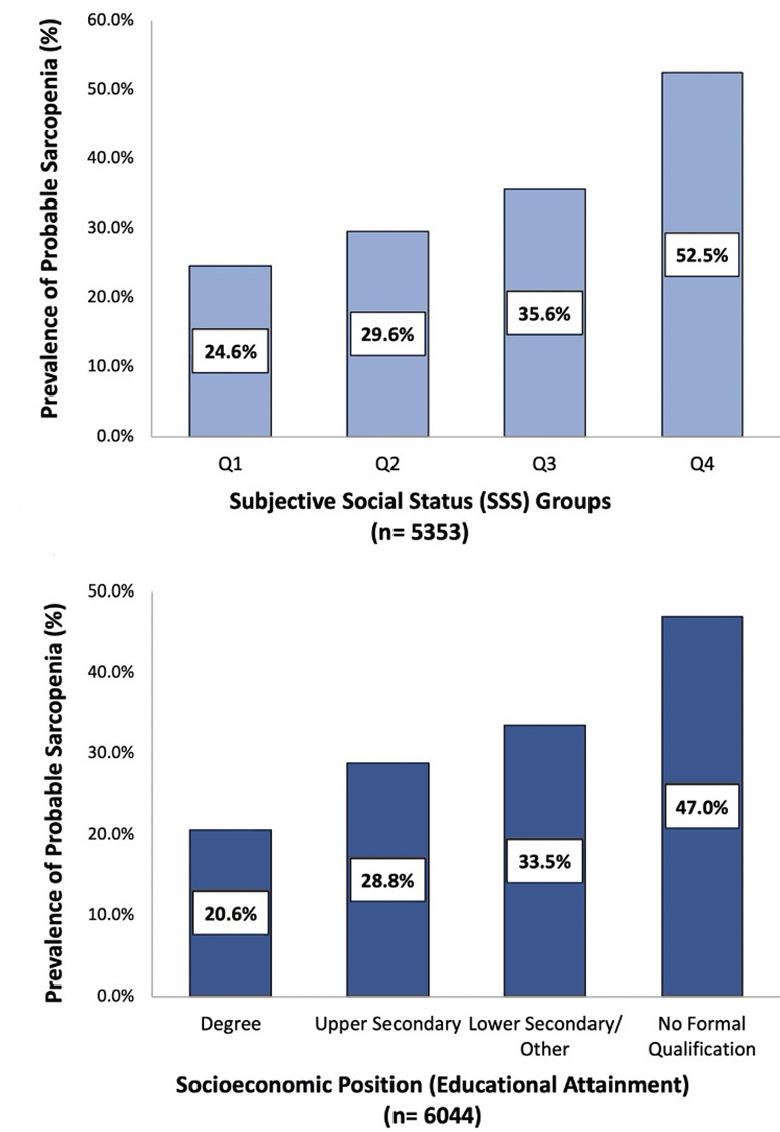
Figure 2. Crude prevalence (%) of probable sarcopenia in community-dwelling older adults based on subjective social status group and socioeconomic position (educational attainment)
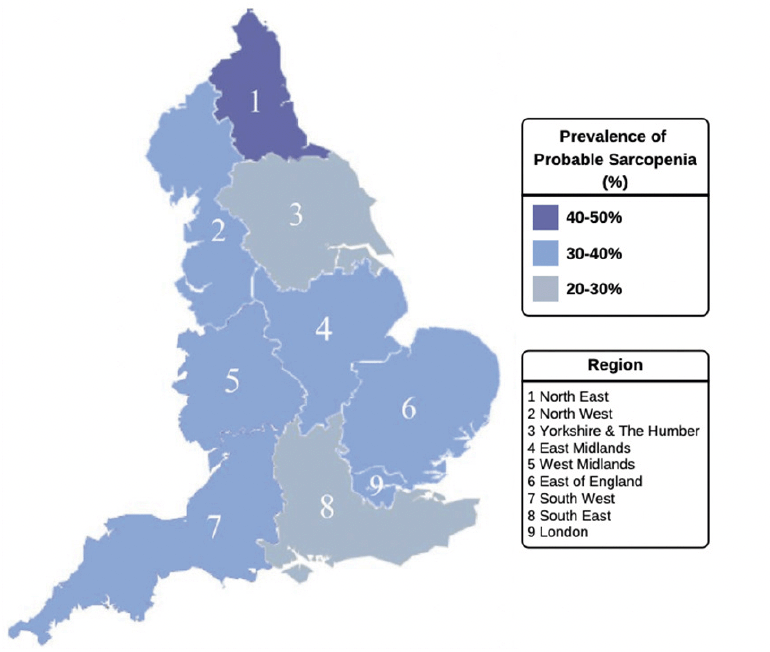
Figure 3. Probable sarcopenia, defined by EWGSOP2 guidelines, in community-dwelling older adults by geographic region in England (n= 6030)
Overall, 18.0% of the population met the criteria for probable sarcopenia based on low hand grip strength alone (weighted 19.9%) and 26.1% by poor chair-rise test performance alone (weighted 28.5%). There was some degree of intersectionality with 26.4% of those with probable sarcopenia meeting the criteria for both low hand grip strength and poor chair rise test performance.
Characteristics of older adults with probable sarcopenia
The characteristics of older adults with probable sarcopenia compared with those who did not meet the EWGSOP2 criteria (reference group) are described in Table 1. Significantly more older adults with probable sarcopenia reported no formal education (38.3% v 21.9%, p<0.001) and fewer reported completion of a third-level qualification (11.0% vs. 21.5%, p<0.001) compared with the reference group, respectively.
The probable sarcopenia group was significantly older (75.6 ± 8.2 vs reference 68.9 ± 6.7, p<0.001) with a higher proportion of females (58.9% vs 52.2%. p<0.001). Physical inactivity (46.9% vs 14.0%, p<0.001), obesity (34.1% vs 29.2%, p<0.001), central adiposity (60.2% vs 51.7%, p<0.001), co-morbidity (68.3% vs 37.0%, <0.001) and osteoarthritis (38.5% vs 27.1%, p<0.001) were significantly more common in the probable sarcopenia group compared with those with preserved muscle strength. Underweight (BMI ≤18.5 kg/m2) was higher in probable sarcopenia relative to the reference group (1.6% vs 0.6%, p<0.001), though the number of participants classified as underweight in the study population overall was small (n= 53). No significant differences in probable sarcopenia were noted on analysis by ethnicity, however, the population sample was not ethnically diverse (n= 141, 2.3% ethnic minority group).
Determinants of probable sarcopenia and markers of low muscle strength – weighted multivariable regression analyses
Pre-selected risk factors of probable sarcopenia were entered into the logistic regression model. In the model, associations persisted between probable sarcopenia and the following predictors: disadvantaged SEP (educational attainment and SSS), older age, physical inactivity, a greater number of chronic conditions, osteoarthritis, and minority group ethnicity (Table 2). Older adults with the most disadvantaged SEP (no formal qualifications) were 1.67 times more likely to have probable sarcopenia [OR, CI 1.67 (1.27, 2.21), p<0.001] than the least disadvantaged (third level degree). Similarly, disadvantaged SSS predicted probable sarcopenia [OR, CI 1.87 (1.18, 2.96), p=0.008]. The model supported an increased likelihood of probable sarcopenia for physical inactivity [OR, CI 3.37 (2.68, 4.24), p<0.001], older age [OR, CI 1.10 (1.08, 1.11), p<0.001], chronic conditions [OR, CI 1.29 (1.21, 1.37), p<0.001] osteoarthritis [OR, CI 1.35 (1.13, 1.60), p<0.001] and minority ethnicity [OR, CI 1.74 (1.13, 2.69), p= 0.012].
A sub-analysis was performed to explore if makers of SEP remained independent determinants of probable sarcopenia when low muscle strength was identified by low hand grip strength alone or poor chair rise test performance alone (Table 2). Consistent with earlier findings, disadvantaged SEP, measured by educational attainment and SSS, were significantly associated with probable sarcopenia, irrespective of the mode of assessment: low hand grip strength [OR, CI 1.83 (1.28, 2.64), p<0.001 and 2.04 (1.23, 3.39), p=0.006, respectively] and poor chair rise test performance [OR, CI 1.40 (1.03, 1.91), p=0.033 and 1.99 (1.21, 3.28), p=0.007, respectively]. Older age, physical inactivity, chronic conditions, and osteoarthritis remained predictors of probable sarcopenia irrespective of the mode of assessment. The BMI, smoking status, and ethnicity differed based on the marker of low muscle strength applied.
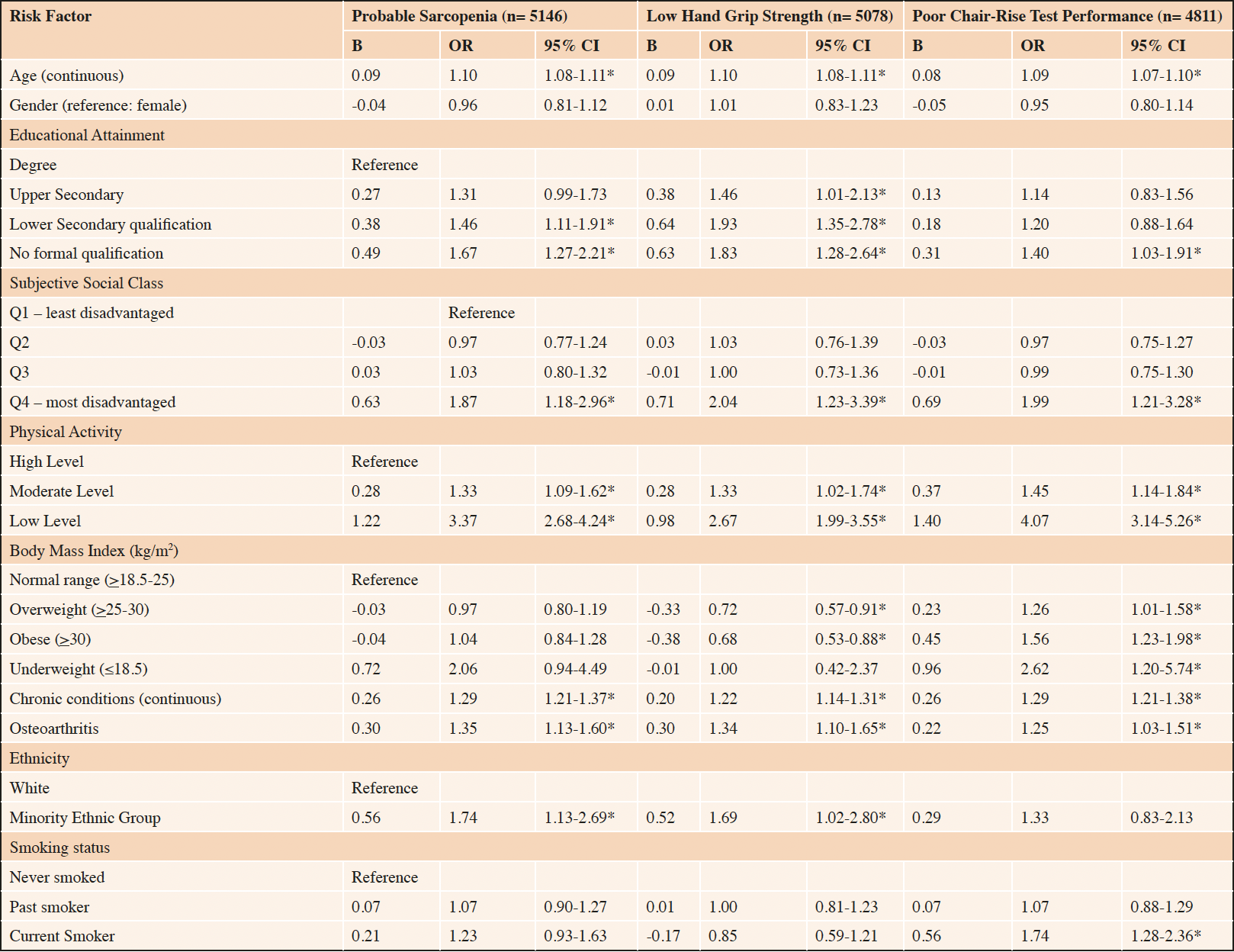
Table 2. Weighted logistic regression model of risk factors of probable sarcopenia (n= 5146), low hand grip strength (n= 5078) and poor chair-rise test performance (n= 4811)
Binary logistic regression analysis was used to determine the Odds Ratio (OR) and 95% Confidence Intervals (CI) for associations between health and sociodemographic variables and probable sarcopenia, low hand grip strength and poor chair rise test performance, respectively. BMI: Body Mass Index; *p<0.05
Discussion
Few studies have investigated socioeconomic determinants of probable sarcopenia. In the present study, we report a 2-fold higher prevalence in older adults with the most, compared with the least, disadvantaged SEP (47% vs 21%, respectively) based on educational attainment and similar findings by subjective social status (53% vs 25%). Furthermore, both markers of disadvantaged SEP were independent determinants of probable sarcopenia when controlled for other known risk factors. The findings are in keeping with the established evidence that socioeconomic disadvantage is associated with negative health outcomes (3–6) and suggest that sarcopenia places an unequal burden on socioeconomically disadvantaged communities. The findings suggest that socioeconomic indicators, in addition to health factors, are important considerations in determining the risk profile and prevention strategies for sarcopenia in older populations
Current evidence remains inconsistent, Pérez-Sousa et al reported a high prevalence of probable sarcopenia (47%) in Colombian older adults (mean age 70 years, n= 5237) and noted that individuals in the most disadvantaged socioeconomic groups had the highest prevalence of probable sarcopenia (76%) (8). Dodds et al, however, observed no association between probable sarcopenia and occupation class in the 1946 British Birth Cohort (18). While these studies provide context, neither study aimed to specifically examine SEP. In contrast, Brennan-Olsen et al, in a study of older adults in Australia across six years, noted a greater decline in hand grip strength and lower limb strength in groups with lower educational attainment and occupation class (9). We previously reported that socioeconomic disadvantage, defined by a single marker of SEP (educational attainment) was an independent predictor of probable sarcopenia in Irish community-dwelling older adults (7). The present study extends this finding to a large population of English older adults with objective and subjective markers of SEP.
The findings suggest a North-South gradient in the regional distribution of probable sarcopenia. This mirrors the growing body of evidence reporting regional health disparities in England, with a greater prevalence of markers of poor health, including frailty, observed among older adults in the North of England (27, 38). The findings, however, are descriptive and would need to account for population demographics and socioeconomic data of the regions, which was beyond the scope of the present study. Future research could include markers of both area-level deprivation along with individual-level SEP when examining probable sarcopenia. As noted in the frailty literature, mapping populations can assist in matching services and resources to peoples’ needs (40). Given that the identification of probable sarcopenia warrants the initiation of interventions, our findings may help inform healthcare policy, service planning and resource allocation to prevent and treat sarcopenia in regions with the greatest socioeconomic need.
There was consistent evidence of significantly greater odds of probable sarcopenia for older adults living with disadvantaged SEP defined by educational attainment or SSS. Older adults with no formal qualifications had 1.7 times greater odds of probable sarcopenia when compared to those with a third-level qualification. Similarly, older adults who perceived their SSS to be disadvantaged relative to society had significantly greater odds of probable sarcopenia. As noted in the sub-analysis, SEP and SSS remained independent determinants of probable sarcopenia in older adults, irrespective of the mode of assessment used to identify low muscle strength. This expands previous work showing associations between SEP and probable sarcopenia defined exclusively by low hand grip strength (7). While the prevalence of probable sarcopenia varies based on the mode of assessment, low hand grip (18%; weighted 20%) and poor chair-rise test (26%; weighted 29%), SEP remains an independent predictor of both measures of low muscle strength.
The findings are in line with previous research which has reported links between disadvantaged SSS and greater functional decline in older adults (41). Moreover, SSS has been identified as an important predictor of mortality in participants of ELSA (32). Similarly, low educational attainment has been linked with weaker hand grip strength across the life course (42). With accumulating evidence suggesting an association between SEP and low muscle strength, the inclusion of markers of SEP in further research and prevention strategies for probable sarcopenia warrants attention (6–8).
Other determinants of probable sarcopenia included older age, physical inactivity, chronic conditions and osteoarthritis. Physical inactivity is a well-documented modifiable risk factor for sarcopenia (18, 43). Minority group ethnicity was a significant determinant of probable sarcopenia, which has been previously suggested (44, 45). This cohort, however, was not ethnically diverse, and most participants were white (97.7%). There is a need to investigate ethnic, as well as socioeconomic, differences in probable sarcopenia given that most studies to date have been conducted on White/Caucasian older adults populations (7, 18, 46, 47). Greater diversity would benefit future large scale ageing studies.
This study has several strengths and limitations. The findings are derived from the English Longitudinal Study of Ageing (ELSA), which is a large nationally representative study of community-dwelling older adults in England. In addition to markers of socioeconomic disadvantage, geographic region of residence and ethnicity were included. The highest formal educational qualification completed was operationalized as a marker of SEP, which has previously been shown as a robust indicator among older adults (48). Ideally, future studies would include a marker of area deprivation in addition to individual-level SEP. Other information relevant to sarcopenia such as nutritional status and protein intake may benefit future studies (49–51). Physical activity level is self-reported which may be influenced by recall bias, however, the threshold selected for each activity level has shown associations with mortality in ELSA participants (51). Essentially, this study was cross-sectional in design and therefore, cannot show cause and effect for the higher prevalence of probable sarcopenia in older adults with disadvantaged SEP. Many studies in this area are not ethnically diverse, and in the present study minority group ethnicity was low (2.3%) (7, 18, 45, 46). Future studies are needed that are more socioeconomically and ethnically diverse and include underserved groups in research.
The present study identified probable sarcopenia in 34% of community-dwelling English older adults (weighted 36%). The etiology of sarcopenia is complex, probable sarcopenia represents a practical and measurable target (e.g. muscle strength), it represents a point to intervene, and the ‘prescription’ treatment is exercise and diet (10). Across the life course, socioeconomic disadvantage is associated with acquiring lower peak muscle strength in adulthood and experiencing earlier decline, compared with less disadvantaged communities (41, 52). Recently, Guo et al, reported that higher educational attainment was associated with a slower decline in markers of muscle mass, in a 15-year longitudinal study (53). The physiological mechanisms underpinning the links between SEP and sarcopenia were beyond the scope of the present study, but current evidence suggests that socioeconomic disadvantage is associated with mediators of chronic disease, including inadequate physical activity, inflammation, poor nutritional status, environmental and occupational hazards, and inaccessibility of health care (1, 54, 55). Importantly, evidence of a substantial socioeconomic gradient throughout the life course has also been shown for physical activity (56).
While our findings suggest that socioeconomic disadvantage is associated with greater odds of sarcopenia, previous research has suggested disadvantaged SEP may impede engagement with physical activity and adequate diet, which are the primary treatment and preventative prescription for sarcopenia (42, 56). Socioeconomic indicators, in addition to health variables, would assist understanding sarcopenia, to develop more inclusive and targeted prevention strategies. These are important considerations given that probable sarcopenia and confirmed sarcopenia have been recently characterized as dynamic and reversible conditions (57).
Conclusion
The findings suggest a high burden of probable sarcopenia among older populations living with socioeconomic disadvantage. In the present study, 47% and 53% of older adults with disadvantaged SEP had probable sarcopenia, assessed by educational attainment and by subjective social status, respectively. Disadvantaged SEP was an independent determinant of probable sarcopenia, and this finding was consistent for both measures of SEP and irrespective of how probable sarcopenia was measured. The results support associations between probable sarcopenia and other risk factors including physical inactivity, older age, chronic conditions, osteoarthritis, and ethnicity. The findings highlight a need and opportunity to address socioeconomic disadvantage in research, policy and practice for sarcopenia prevention and treatment.
Acknowledgements: We would like to thank all participants of the English Longitudinal Study of Ageing.
Funding: This study was part-funded by North Dublin Home Care, a non-profit organization in Ireland (Registered Charity Number: 20076245). North Dublin Homecare provide a PhD funding bursary for author LS only and had no role in the design of the study; in the collection, analyses, or interpretation of data; in the preparation of the manuscript, or in the review or approval of the manuscript and in the decision to publish the results. Open Access funding provided by the IReL Consortium.
Conflict of Interest: All authors declare no conflict of interest.
Ethical standard: This study was carried out in accordance with ethical standards. Ethical approval was granted by the National Research Ethics Service (MREC/01/2/91).
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Murray CJL, Abbafati C, Abbas KM, Abbasi M, Abbasi-Kangevari M, Abd-Allah F, et al. Five insights from the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1135‑59. DOI: 10.1016/S0140-6736(20)31404-5
2. Marmot M. Social determinants of health inequalities. The Lancet. Elsevier; 2005;365(9464):1099‑104. DOI: 10.1016/S0140-6736(05)71146-6
3. Chan MS, van den Hout A, Pujades-Rodriguez M, Jones MM, Matthews FE, Jagger C, et al. Socio-economic inequalities in life expectancy of older adults with and without multimorbidity: a record linkage study of 1.1 million people in England. Int J Epidemiol. 2019;48(4):1340‑51. DOI: 10.1093/ije/dyz052
4. Head A, Fleming K, Kypridemos C, Schofield P, Pearson-Stuttard J, O’Flaherty M. Inequalities in incident and prevalent multimorbidity in England, 2004–19: a population-based, descriptive study. Lancet Healthy Longev. 2021;2(8):e489‑97. DOI: 10.1016/S2666-7568(21)00146-X
5. McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front Public Health. 2020;8:231. DOI: 10.3389/fpubh.2020.00231
6. Steptoe A, Zaninotto P. Lower socioeconomic status and the acceleration of aging: An outcome-wide analysis. Proc Natl Acad Sci U S A. 2020;117(26):14911‑7. DOI: 10.1073/pnas.1915741117
7. Swan L, Warters A, O’Sullivan M. Socioeconomic Inequality and Risk of Sarcopenia in Community-Dwelling Older Adults. Clin Interv Aging. Dove Press; 2021;16:1119‑29. DOI: 10.2147/CIA.S310774
8. Pérez-Sousa MÁ, Pozo-Cruz J del, Cano-Gutiérrez CA, Izquierdo M, Ramírez-Vélez R. High Prevalence of Probable Sarcopenia in a Representative Sample From Colombia: Implications for Geriatrics in Latin America. J Am Med Dir Assoc. Elsevier; 2020;0(0). DOI: 10.1016/j.jamda.2020.10.021
9. Brennan-Olsen SL, Vogrin S, Balogun S, Wu F, Scott D, Jones G, et al. Education, occupation and operational measures of sarcopenia: Six years of Australian data. Australas J Ageing. 2020;39(4):e498‑505. DOI: 10.1111/ajag.12816
10. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‑31. DOI: 10.1093/ageing/afy169
11. Xu W, Chen T, Cai Y, Hu Y, Fan L, Wu C. Sarcopenia in Community-Dwelling Oldest Old Is Associated with Disability and Poor Physical Function. J Nutr Health Aging. 2020;24(23):339‑45. DOI: 10.1007/s12603-020-1325-4
12. Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;8(2):251‑8. DOI: 10.1002/jcsm.12163
13. Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. 2018;18. DOI: 10.1186/s12877-018-0878-0
14. Ibrahim K, May C, Patel HP, Baxter M, Sayer AA, Roberts H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): study protocol. Pilot Feasibility Stud. 2016;2. DOI: 10.1186/s40814-016-0067-x
15. Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Lond Engl. 2015;386(9990):266‑73. DOI: 10.1016/S0140-6736(14)62000-6
16. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73(9):1199‑204. DOI: 10.1093/gerona/glx245
17. Dodds RM, Granic A, Robinson SM, Sayer AA. Sarcopenia, long-term conditions, and multimorbidity: findings from UK Biobank participants. J Cachexia Sarcopenia Muscle. 2020;11(1):62‑8. DOI: 10.1002/jcsm.12503
18. Dodds RM, Murray JC, Robinson SM, Sayer AA. The identification of probable sarcopenia in early old age based on the SARC-F tool and clinical suspicion: findings from the 1946 British birth cohort. Eur Geriatr Med. 2020;11(3):433‑41. DOI: 10.1007/s41999-020-00310-5
19. Kim M, Won CW. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing. Oxford Academic; 2019;48(6):910‑6. DOI: 10.1093/ageing/afz091
20. Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):318. DOI: 10.1186/s12877-019-1338-1
21. Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007;81‑82(1):21‑37. DOI: 10.1093/bmb/ldm001
22. Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341‑78. DOI: 10.1146/annurev.publhealth.18.1.341
23. Fiorito G, McCrory C, Robinson O, Carmeli C, Rosales CO, Zhang Y, et al. Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi-cohort analysis. Aging. 2019;11(7):2045‑70. DOI: 10.18632/aging.101900
24. Stringhini S, Carmeli C, Jokela M, Avendaño M, McCrory C, d’Errico A, et al. Socioeconomic status, non-communicable disease risk factors, and walking speed in older adults: multi-cohort population based study. BMJ. British Medical Journal Publishing Group; 2018;360. DOI: 10.1136/bmj.k1046
25. Demakakos P, Marmot M, Steptoe A. Socioeconomic position and the incidence of type 2 diabetes: the ELSA study. Eur J Epidemiol. 2012;27(5):367‑78. DOI: 10.1007/s10654-012-9688-4
26. Corris V, Dormer E, Brown A, Whitty P, Collingwood P, Bambra C, et al. Health inequalities are worsening in the North East of England. Br Med Bull. 2020;134(1):63‑72. DOI: 10.1093/bmb/ldaa008
27. Sinclair DR, Maharani A, Chandola T, Bower P, Hanratty B, Nazroo J, et al. Frailty among Older Adults and Its Distribution in England. J Frailty Aging. 2021; DOI: 10.14283/jfa.2021.55
28. Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42(6):1640‑8. DOI: 10.1093/ije/dys168
29. Dodds RM, Pakpahan E, Granic A, Davies K, Sayer AA. The recent secular trend in grip strength among older adults: findings from the English Longitudinal Study of Ageing. Eur Geriatr Med. 2019;10(3):395‑401. DOI: 10.1007/s41999-019-00174-4
30. Ploubidis GB, DeStavola BL, Grundy E. Health differentials in the older population of England: An empirical comparison of the materialist, lifestyle and psychosocial hypotheses. BMC Public Health. 2011;11(1):390. DOI: 10.1186/1471-2458-11-390
31. Tsimpida D, Kontopantelis E, Ashcroft D, Panagioti M. Socioeconomic and lifestyle factors associated with hearing loss in older adults: a cross-sectional study of the English Longitudinal Study of Ageing (ELSA). BMJ Open. British Medical Journal Publishing Group; 2019;9(9):e031030. DOI: 10.1136/bmjopen-2019-031030
32. Demakakos P, Biddulph JP, de Oliveira C, Tsakos G, Marmot MG. Subjective social status and mortality: the English Longitudinal Study of Ageing. Eur J Epidemiol. 2018;33(8):729‑39. DOI: 10.1007/s10654-018-0410-z
33. Rosário EVN, Severo M, Francisco D, Brito M, Costa D. Examining the relation between the subjective and objective social status with health reported needs and health-seeking behaviour in Dande, Angola. BMC Public Health. 2021;21(1):979. DOI: 10.1186/s12889-021-11003-4
34. Smith L, Gardner B, Fisher A, Hamer M. Patterns and correlates of physical activity behaviour over 10 years in older adults: prospective analyses from the English Longitudinal Study of Ageing. BMJ Open. British Medical Journal Publishing Group; 2015;5(4):e007423. DOI: 10.1136/bmjopen-2014-007423
35. Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595‑602. DOI: 10.1016/j.jclinepi.2004.10.018
36. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. World Health Organization; 2000.
37. Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. BMJ Publishing Group; 1995;311(6998):158. DOI: 10.1136/bmj.311.6998.158
38. Batty D, Blake M, Bridges S, Crawford R, Demakakos P, Oliveira C de, et al. The Dynamics of ageing: Evidence from the English Longitudinal Study of Ageing 2002-2016 (Wave 6). The IFS; 15 octobre 2018 [cité le 4 février 2021]. Disponible: https://www.ifs.org.uk/uploads/ELSA_Wave_8_report.pdf
39. Aspell N, Laird E, Healy M, Shannon T, Lawlor B, O’Sullivan M. The Prevalence and Determinants of Vitamin D Status in Community-Dwelling Older Adults: Results from the English Longitudinal Study of Ageing (ELSA). Nutrients. 2019;11(6):1253. DOI: 10.3390/nu11061253
40. Taylor D, Barrie H, Lange J, Thompson MQ, Theou O, Visvanathan R. Geospatial modelling of the prevalence and changing distribution of frailty in Australia – 2011 to 2027. Exp Gerontol. 2019;123:57‑65. DOI: 10.1016/j.exger.2019.05.010
41. Chen B, Covinsky KE, Cenzer IS, Adler N, Williams BA. Subjective Social Status and Functional Decline in Older Adults. J Gen Intern Med. 2012;27(6):693‑9. DOI: 10.1007/s11606-011-1963-7
42. Carney C, Benzeval M. Social patterning in grip strength and in its association with age; a cross sectional analysis using the UK Household Longitudinal Study (UKHLS). BMC Public Health. 2018;18(1):385. DOI: 10.1186/s12889-018-5316-x
43. Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25(7):824‑53. DOI: 10.1007/s12603-021-1665-8
44. Dorhout BG, Overdevest E, Tieland M, Nicolaou M, Weijs PJM, Snijder MB, et al. Sarcopenia and its relation to protein intake across older ethnic populations in the Netherlands: the HELIUS study. Ethn Health. Taylor & Francis; 2020;0(0):1‑16. DOI: 10.1080/13557858.2020.1814207
45. Duchowny KA, Hicken MT, Cawthon PM, Glymour MM, Clarke P. Life course trauma and muscle weakness in older adults by gender and race/ethnicity: Results from the U.S. health and Retirement Study. SSM – Popul Health. 2020;11. DOI: 10.1016/j.ssmph.2020.100587
46. Bertschi D, Kiss CM, Beerli N, Kressig RW. Sarcopenia in hospitalized geriatric patients: insights into prevalence and associated parameters using new EWGSOP2 guidelines. Eur J Clin Nutr. 2021;75(4):653‑60. DOI: 10.1038/s41430-020-00780-7
47. Martone AM, Marzetti E, Salini S, Zazzara MB, Santoro L, Tosato M, et al. Sarcopenia Identified According to the EWGSOP2 Definition in Community-Living People: Prevalence and Clinical Features. J Am Med Dir Assoc. 2020;21(10):1470‑4. DOI: 10.1016/j.jamda.2020.03.007
48. Grundy E, Holt G. The socioeconomic status of older adults: How should we measure it in studies of health inequalities? J Epidemiol Community Health. 2001;55(12):895‑904. DOI: 10.1136/jech.55.12.895
49. Aspell N, Laird E, Healy M, Lawlor B, O’Sullivan M. Vitamin D Deficiency Is Associated With Impaired Muscle Strength And Physical Performance In Community-Dwelling Older Adults: Findings From The English Longitudinal Study Of Ageing. Clin Interv Aging. 2019;14:1751‑61. DOI: 10.2147/CIA.S222143
50. Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2013;28(6):684‑90. DOI: 10.1177/0884533613507607
51. Kwon Y-J, Kim HS, Jung D-H, Kim J-K. Cluster analysis of nutritional factors associated with low muscle mass index in middle-aged and older adults. Clin Nutr. Elsevier; 2020;39(11):3369‑76. DOI: 10.1016/j.clnu.2020.02.024
52. Hamer M, de Oliveira C, Demakakos P. Non-exercise physical activity and survival: English longitudinal study of ageing. Am J Prev Med. 2014;47(4):452‑60. DOI: 10.1016/j.amepre.2014.05.044
53. Pengpid S, Peltzer K. Hand Grip Strength and Its Sociodemographic and Health Correlates among Older Adult Men and Women (50 Years and Older) in Indonesia. Curr Gerontol Geriatr Res. Hindawi; 2018;2018:e3265041. DOI: 10.1155/2018/3265041
54. Guo J, Shang Y, Fratiglioni L, Johnell K, Welmer A-K, Marseglia A, et al. Individual changes in anthropometric measures after age 60 years: a 15-year longitudinal population-based study. Age Ageing. 2021;50(5):1666‑74. DOI: 10.1093/ageing/afab045
55. Marmoth, Richardson. Social Determinants of Health [En ligne]. Oxford University Press; 2005 [cité le 20 août 2019]. Disponible: https://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780198565895.001.0001/acprof-9780198565895
56. Wang J, Geng L. Effects of Socioeconomic Status on Physical and Psychological Health: Lifestyle as a Mediator. Int J Environ Res Public Health. 2019;16(2). DOI: 10.3390/ijerph16020281
57. Farrell L, Hollingsworth B, Propper C, Shields MA. The socioeconomic gradient in physical inactivity: Evidence from one million adults in England. Soc Sci Med. 2014;123:55‑63. DOI: 10.1016/j.socscimed.2014.10.039
58. Trevisan C, Vetrano DL, Calvani R, Picca A, Welmer A-K. Twelve-year sarcopenia trajectories in older adults: results from a population-based study. J Cachexia Sarcopenia Muscle. 2021; DOI: 10.1002/jcsm.12875