S.R. Bauer1,2,3, C.E. McCulloch4, P.M. Cawthon4,5, K.E. Ensrud6, A.M. Suskind2, J.C. Newman7,9, S.L. Harrison5, A. Senders8, K. Covinsky3,9, L.M. Marshall8 for the Osteoporotic Fractures in Men (MrOS) Research Group
1. Division of General Internal Medicine, Department of Medicine, University of California, San Francisco, CA, USA; 2. Department of Urology, University of California, San Francisco, CA, USA; 3. San Francisco VA Healthcare System, San Francisco, CA, USA; 4. Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA;
5. Research Institute, California Pacific Medical Center, San Francisco, CA, USA; 6. Department of Medicine and Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA; 7. Buck Institute for Research on Aging, Novato, CA, USA; 8. Oregon Health and Science University-Portland State University School of Public Health, Portland, OR, USA; 9. Division of Geriatrics, Department of Medicine, University of California, San Francisco, CA, USA
Corresponding Author: Scott R. Bauer, SFVA Medical Center, Division of General Internal Medicine 4150 Clement St., Building 2, Room 135, San Francisco, CA 94121, USA, Email: Scott.Bauer@ucsf.edu, Phone: 415-221-4810 x24322, Twitter handle: @ScottBauerMD, Publicly available data: https://mrosonline.ucsf.edu
J Frailty Aging 2023;12(2)117-125
Published online April 22, 2022, http://dx.doi.org/10.14283/jfa.2022.33
Abstract
Background: Lower urinary tract symptoms (LUTS) are associated with prevalent frailty and functional impairment, but longitudinal associations remain unexplored.
Objectives: To assess the association of change in phenotypic frailty with concurrent worsening LUTS severity among older men without clinically significant LUTS at baseline.
Design: Multicenter, prospective cohort study.
Setting: Population-based.
Participants: Participants included community-dwelling men age ≥65 years at enrollment in the Osteoporotic Fractures in Men study.
Measurements: Data were collected at 4 visits over 7 years. Phenotypic frailty score (range: 0-5) was defined at each visit using adapted Fried criterion and men were categorized at baseline as robust (0), pre-frail (1-2), or frail (3-5). Within-person change in frailty was calculated at each visit as the absolute difference in number of criteria met compared to baseline. LUTS severity was defined using the American Urologic Association Symptom Index (AUASI; range: 0-35) and men with AUASI ≥8 at baseline were excluded. Linear mixed effects models were adjusted for demographics, health-behaviors, and comorbidities to quantify the association between within-person change in frailty and AUASI.
Results: Among 3235 men included in analysis, 48% were robust, 45% were pre-frail, and 7% were frail. Whereas baseline frailty status was not associated with change in LUTS severity, within-person increases in frailty were associated with greater LUTS severity (quadratic P<0.001). Among robust men at baseline, mean predicted AUASI during follow-up was 4.2 (95% CI 3.9, 4.5) among those meeting 0 frailty criteria, 4.6 (95% CI 4.3, 4.9) among those meeting 1 criterion increasing non-linearly to 11.2 (95% CI 9.8, 12.6) among those meeting 5 criteria.
Conclusions: Greater phenotypic frailty was associated with non-linear increases in LUTS severity in older men over time, independent of age and comorbidities. Results suggest LUTS and frailty share an underlying mechanism that is not targeted by existing LUTS interventions.
Key words: Aging, epidemiology, benign prostatic hyperplasia, sarcopenia.
Introduction
Lower urinary tract symptoms (LUTS) increase dramatically with age and almost half of men will be affected after age 70 (1, 2). LUTS is a constellation of frequently overlapping symptoms that occur when urine is generated and stored in the bladder, called storage LUTS (e.g., urgency, frequency, nocturia, and urinary incontinence), during the initiation and process of urination, called voiding LUTS (e.g., weak stream, hesitancy, straining, and incomplete bladder emptying), or immediately after voiding (e.g. post-void dribbling) (3). Older men with LUTS are more likely to be phenotypically frail (4) and functionally impaired (2) and, in some but not all studies, have increased risk of falls, fractures, and death (5-7). The most common male LUTS treatments narrowly target urologic pathology (α-blockers, 5α-reductase inhibitors, and anti-muscarinics) and are independently associated with increased risk of incident falls and fractures (8), depression and suicidal ideation (9), and dementia (10). Despite evidence that both LUTS and existing LUTS treatments are associated with major geriatric conditions, only urinary incontinence, the most bothersome form of LUTS for most adults (11), is considered a geriatric syndrome and some professional societies recommend that older adults with urinary incontinence undergo a comprehensive geriatric assessment and multicomponent intervention (12-14). It remains unknown if older men with other LUTS subtypes may benefit from a more holistic diagnostic and management approach as well.
Male LUTS are frequently attributed to bladder outlet obstruction due to benign prostatic hyperplasia (BPH). However, there are several non-urologic and systemic factors that contribute to LUTS, especially among older men (15). In fact, men with severe LUTS are only 50% more likely to have bladder outlet obstruction confirmed via urodynamics and men with moderate LUTS have the same likelihood of bladder outlet obstruction as those without LUTS (16). The presence of LUTS is similarly a weak predictor of abnormal bladder contractions detected via urodynamics, such as detrusor overactivity (17). These observations have led to the hypothesis that there are alternative, age-related mechanisms of LUTS that are not targeted by existing therapies. Novel therapies targeting these mechanisms, such as frailty, sarcopenia, or altered circadian rhythm, may reduce both symptom severity and the risk of co-occurring geriatric syndromes (4). Although LUTS are cross-sectionally associated with phenotypic frailty, it remains unknown if older men develop phenotypic frailty and LUTS concurrently.
To address this gap in knowledge, we evaluated the association of change in phenotypic frailty with concurrent change in LUTS severity, overall and by storage and voiding subscores, in a large, prospective cohort of older, community-dwelling men without clinically significant LUTS at baseline. We hypothesized that men who become more frail, as manifested by a greater number of phenotypic frailty components, will also have increasing LUTS severity.
Methods
Participants
The Osteoporotic Fractures in Men (MrOS) study is a large, multicenter cohort study of 5,994 community-dwelling men age 65 years or older as previously described (18, 19). Briefly, this cohort was designed to collect comprehensive data to study older men’s health, including urologic symptoms, with a particular focus on falls and fractures. Men were recruited from March 2000 to April 2002 from six academic medical centers in Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. All eligible surviving participants were invited to complete a questionnaire during Year 2 (July 2002 – March 2004) and to return to the clinic during Year 5 (March 2005 – May 2006) and Year 7 (March 2007 – March 2009). The analytic cohort included 3235 men who completed the LUTS questionnaire and at least 3 frailty phenotype components assessed at baseline, and who initially reported none/mild LUTS severity (AUASI<8) (Supplemental Figure 1). All participants gave written informed consent and Institutional Review Boards at each participating institution approved the study.
LUTS Assessment
LUTS were assessed at 4 time points using the validated and widely used 7-item American Urological Association Symptom Index (AUASI) (20), including individual items on urinary frequency, urgency, intermittency, straining, weak urinary stream, incomplete bladder emptying, and nocturia. Responses to each item are on an ordinal scale with values ranging from 0 to 5, with 0 representing no symptoms and 5 representing the highest symptom burden; total scores range from 0 to 35. For example, to evaluate the storage symptom of urgency men were asked “Over the past month, how often have you found it difficult to postpone urination?” and to evaluate the voiding symptom of incomplete emptying men were asked “Over the past month, how often have you had a sensation of not emptying your bladder completely after you finish urinating?” Response options included “Not at all”, “Less than 1 time in 5”, “Less than half the time”, “About half the time”, “More than half the time”, or “Almost always”. The AUASI has clinically relevant categories of 0 to 7 (none/mild), 8 to 19 (moderate), and 20 to 35 (severe) (21) and the minimal clinically important difference is 3 points (22). In addition to the total score, we calculated AUASI subscores separately for storage symptoms (urgency, frequency, nocturia) and for voiding symptoms (incomplete emptying, intermittency, weak stream, straining), consistent with the literature (23).
Other Measurements
Age, race/ethnicity and education were assessed via self-administered questionnaires at baseline, and marital status, smoking status, and usual alcohol consumption were updated via self-administered questionnaires at every study visit.18 Participants reported history of myocardial infarction, angina, heart failure, hypertension, diabetes, prostate cancer and prostate cancer treatments (51% treated with surgery, 29% with radiation only, 14% with hormones only, and 6% were not treated), stroke, Parkinson’s disease, osteoporosis, osteoarthritis, chronic obstructive pulmonary disease, and thyroid disease. Multimorbidity was defined as the cumulative number of 10 most common chronic diseases listed above (24). All participants completed the Medical Outcomes Study Short Form (SF-12) and the mental health component score ≤50 was used as a measure of psychological distress (25). Cognitive function was assessed using the Modified Mini Mental State Examination (3MS) and cognitive impairment was defined as 3MS<80 (26). Comprehensive prescription medication use was coded from labels on pill packets and canisters brought in by the participant, and medications to treat LUTS (α-antagonist, 5α-reductase, or anti-cholinergic) were identified using the Iowa Drug Information System (IDIS) (27). Men were asked if a doctor had told them they “have or had an enlarged prostate (benign prostatic hyperplasia)” and if so, they were asked if they received “Surgery” for this condition, which was used to define self-reported BPH surgery.
Phenotypic Frailty Component Measurements
For determining frailty status, physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) (28). Tests of physical function included maximum grip strength (measured bilaterally using a hand-held Jamar dynamometer) and walk speed (time in seconds to walk 6 meters at usual pace expressed as m/sec). Study staff measured height at each visit using wall-mounted Harpenden stadiometers. Weight was measured with a digital scale or with a standard regularly calibrated balance beam scale. Height and weight measurements were used to calculate a standard body mass index (BMI). Appendicular skeletal muscle mass, as the measure of lean mass, as well as body fat were determined using dual-energy x-ray absorptiometry (DXA; Hologic QDR4500W scanners, Hologic Inc., Bedford, MA) using standardized scanning procedures.
Assessment of Phenotypic Frailty
We used the framework of phenotypic frailty proposed by Fried et al (29, 30) adapted for the MrOS cohort (31). The following frailty phenotype components were assessed at baseline and 2 subsequent follow-up visits :
1. Shrinking/Sarcopenia, identified by an appendicular lean mass (adjusted for height and total body fat) in the lowest quintile;
2. Weakness, identified by a grip strength in the lowest quintile stratified by BMI (quartiles);
3. Exhaustion, identified at baseline by an answer of “a little or none” to the question “How much of the time during the past four weeks did you have a lot of energy?” from the SF-12 and identified at Visit 2 and 3 by an answer of “no” to the question “Do you feel full of energy?” from the Geriatric Depression Scale;
4. Slowness, identified by a walk speed in the lowest quintile stratified by standing height (median); and
5. Low physical activity, level as identified by a PASE score in the lowest quintile.
Frailty criteria at the follow-up examination were defined using the same cut-points as the baseline examination. Men who met ≥3 criteria were considered frail, those who met 1 or 2 criteria were considered pre-frail, and those who met none of the above criteria were considered robust.
Statistical Analysis
In this analytic cohort, defined in part by the absence of moderate-to-severe LUTS at baseline, the primary independent variable was within-person change in phenotypic frailty score and the primary dependent variable was LUTS severity based on AUASI score (total, storage subscore, and voiding subscore) at each repeated assessment. We first compared distributions of established frailty and LUTS risk factors across categories of frailty phenotype. To test the hypothesis that greater baseline phenotypic frailty is associated with greater annual increases in AUASI score, we used linear mixed effect models and modeled phenotypic frailty categories to represent between-person differences. We then used linear mixed effect models with age as the time variable to test the hypothesis that within-person changes in phenotypic frailty score are associated with concurrent changes in AUASI score because within-person changes are less susceptible to confounding due to characteristics that do not vary over time (32). To represent between-person differences, we included a continuous variable for baseline phenotypic frailty score, and to represent within-person changes, we included a continuous time-varying variable for change in phenotypic frailty score (measurement at each visit minus measurement at baseline) (32). All linear mixed models included random intercepts and slopes and used an unstructured variance-covariance matrix. To visualize the trajectory of AUASI scores over time according to within-person changes in frailty, we created a plot of predicted AUASI scores by within-person change in phenotypic frailty score. For this plot, we set age to the median (73 years), baseline frailty phenotype score to the median (1), and all other covariates to 0.
To identify and control for confounding factors, we applied a change in estimate criteria (33). First, we specified variables to be forced into the model (age and study enrollment site) and four groups of potential confounders: demographics (education, race, and marital status), health-related behaviors (smoking and alcohol intake), cardiovascular comorbidities (self-reported history of myocardial infarction, angina, health failure, and hypertension), and other medical comorbidities (and self-reported history of diabetes mellitus, prostate cancer, chronic obstructive pulmonary disease, and stroke or Parkinson’s). Next, we fit a full multivariable model including age, site, and all 4 groups of potential confounders. We then successively removed groups of variables from the full model and each time calculated the % change in the beta coefficient compared to the full model, with a change of ≥10% used to indicate important confounding (all groups met this criteria) (34). Subsequently, we successively removed individual variables from each group until remaining groups only contained variables that contribute ≥1% of the % change for that group of variables. The final multivariable model included age (continuous in years), study site, and self-reported angina, heart failure, hypertension, diabetes mellitus, stroke or Parkinson’s disease, and chronic obstructive pulmonary disease.
We assessed effect modification of the main associations by including a cross product term of the within-person change in phenotypic frailty score by age, LUTS treatment (medication or surgery), neurologic disease (stroke or Parkinson’s disease), or diabetes. For missing data, we conducted pattern mixture models to test for informative dropout due to unmeasured variables. Since we observed no evidence of effect modification or bias due to informative censoring, we report all results for the entire study population. We also conducted sensitivity analyses further adjusting for variables that could be confounders or mediators, including anxiety/depression (SF-12 mental health component score ≤5025), multimorbidity, self-reported general health status, and number of LUTS medications. Lastly, we conducted sensitivity analyses excluding men with urinary incontinence (at least weekly), cognitive impairment (3MS <80), or a baseline phenotypic frailty score of 5 (to minimize ceiling effects) and further adjusting for diuretic medication use.
P-value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Results
General characteristics of the 3235 community-dwelling older men in the analytic cohort are reported in Table 1. In this analytic cohort, 48% of men at baseline were robust (phenotypic frailty score = 0), 45% were pre-frail (phenotypic frailty score median = 1, range 1-2), and 7% were frail (phenotypic frailty score median = 3, range 3-5). Men categorized as frail were older, less likely to be college educated or married, had lower appendicular lean body mass, walking speed, and maximum grip strength, were less likely to report “Excellent” general health status or feeling full of energy, were more sedentary, and had greater burden of comorbidities. Frail men were also more likely to report a history of BPH surgery.
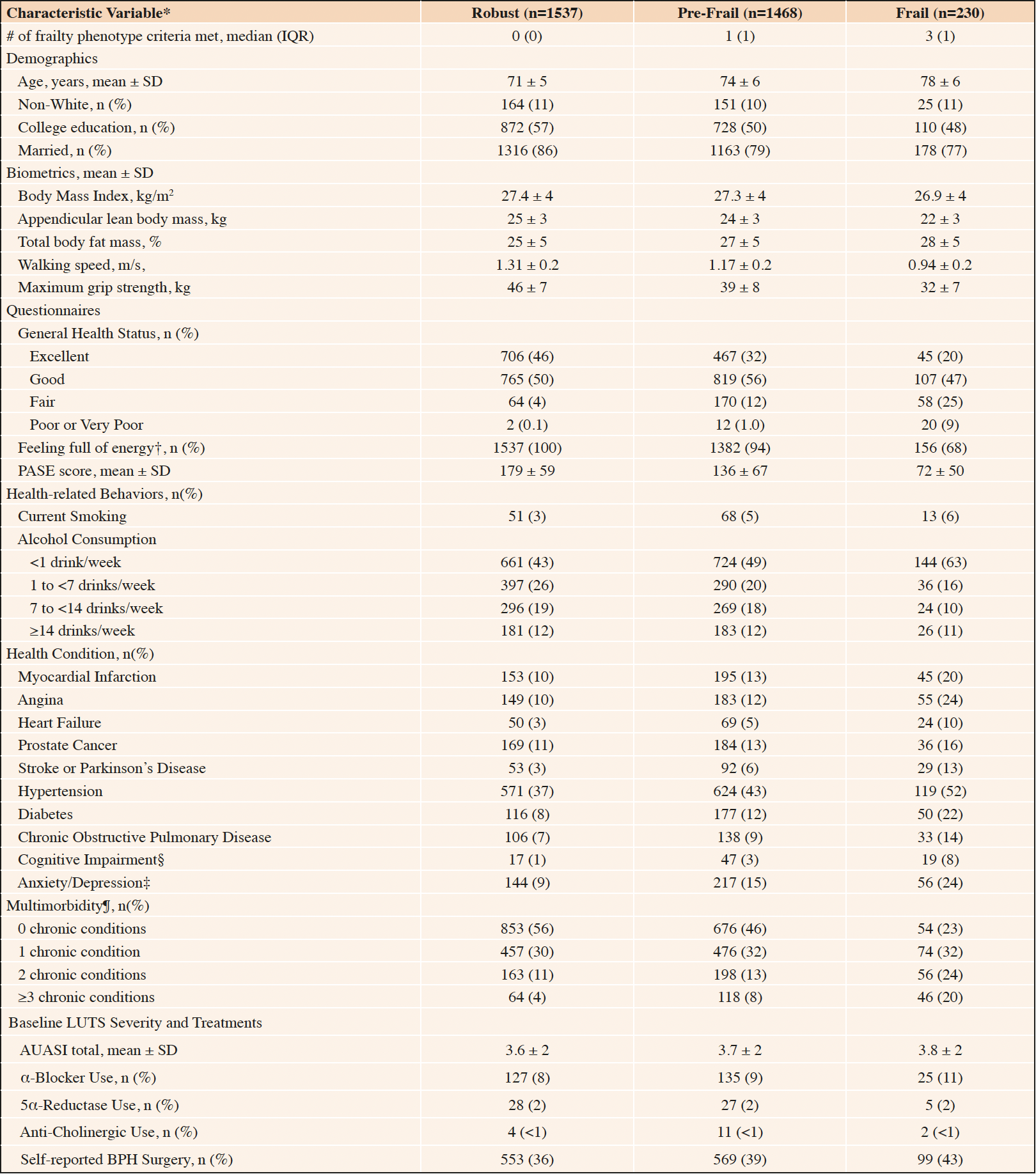
Table 1. General characteristics of men enrolled in MrOS with none/mild lower urinary tract symptoms (LUTS) at baseline, by baseline frailty status
n sample size; IQR interquartile range; SD standard deviation; PASE physical activity scale for the elderly; AUASI American Urological Association Symptom Index; BPH benign prostatic hyperplasia; * Normally distributed continuous variables were reported as mean ± SD, skewed continuous variables were reported as median (IQR), and categorical variables were reported as n (%); † Patients who reported feeling like they “have a lot of energy” at least some of the time; ‡ Short Form-12 Mental Health Component Summary ≤50; § Teng Mini-Mental Status (3MS) <80; ¶ Cumulative number of the following chronic medical conditions: stroke, Parkinson’s disease, myocardial infarction, angina, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, osteoporosis, osteoarthritis, hyperthyroidism or hypothyroidism.
Annual change estimates for AUASI score and age/site-adjusted associations between baseline frailty status and annual change in AUASI are reported in Table 2. Estimated unadjusted annual change in AUASI score was 0.15 (95% 0.13, 0.18) among robust men, 0.18 (95% CI 0.15, 0.20) among pre-frail men, and 0.12 (95% CI 0.06, 0.18) among frail men. In age/site-adjusted models, baseline frailty status was not significantly associated with annual change in AUASI. Baseline frailty status was similarly not associated with change in storage or voiding LUTS subscores.
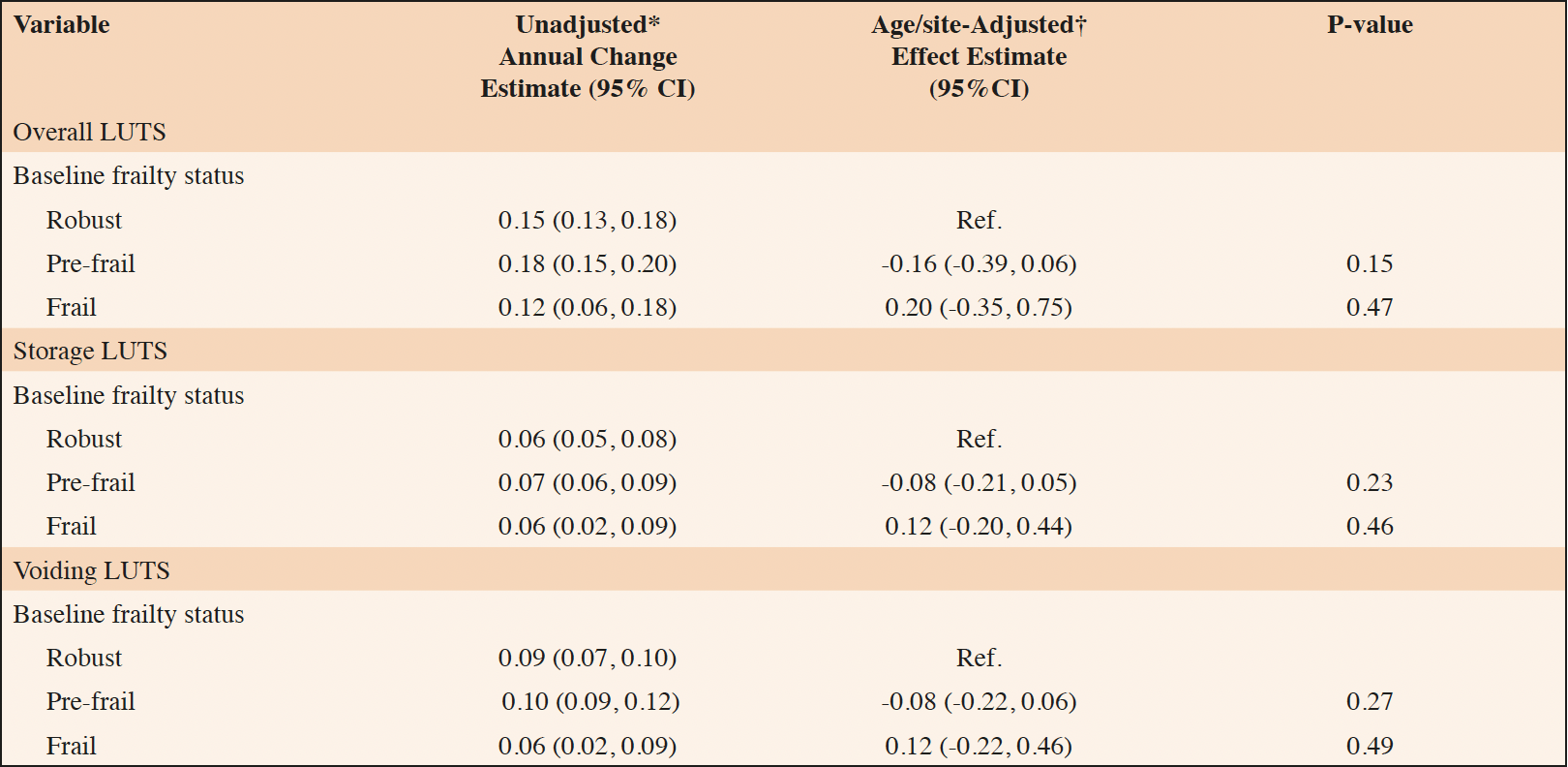
Table 2. Association of baseline frailty status with annual changes in overall, storage, and voiding lower urinary tract symptoms (LUTS)
* Annual change estimate calculated using linear mixed effects models; † Effect estimate calculated using linear mixed effects models. P-value calculated for comparison of annual change among pre-frail and frail men compared to the annual change among robust men.
Predicted AUASI scores by within-person change in phenotypic frailty score during follow-up are reported in Table 3. Among robust men who did not meet any frailty criterion at baseline or during follow-up, mean predicted AUASI was 4.2 (95% CI 3.9, 4.5). Among robust men who developed new pre-frailty during follow-up (meeting an additional 1 or 2 frailty criteria), mean predicted AUASI was 4.6 (95% CI 4.3, 4.9) and 5.6 (95% CI 5.2, 5.9), respectively. Among robust men who developed new frailty (meeting an additional 3 or more frailty criteria) during follow-up, mean predicted AUASI was 7.0 (95% CI 6.4, 7.5) among those with 3 criteria, 8.8 (95% CI 8.0, 9.7) among those with 4 criteria, and 11.2 (95% CI 9.8, 12.6) among those with 5 criteria. Among men who met 1 frailty criterion at baseline and during follow-up, mean predicted AUASI was 4.0 (95% CI 3.7, 4.3) and increased to 8.7 (95% CI 7.8, 9.5) for those who met 4 additional criteria during follow-up. We visualized this concurrent and non-linear increase in AUASI score with increasing frailty scores among men who met 1 frailty phenotype criterion at baseline in Figure 1. Among men with 2 or more frailty criteria at baseline, similar increases in mean predicted AUASI were observed. Regression coefficients for these non-linear associations are reported in Supplemental Table 1.
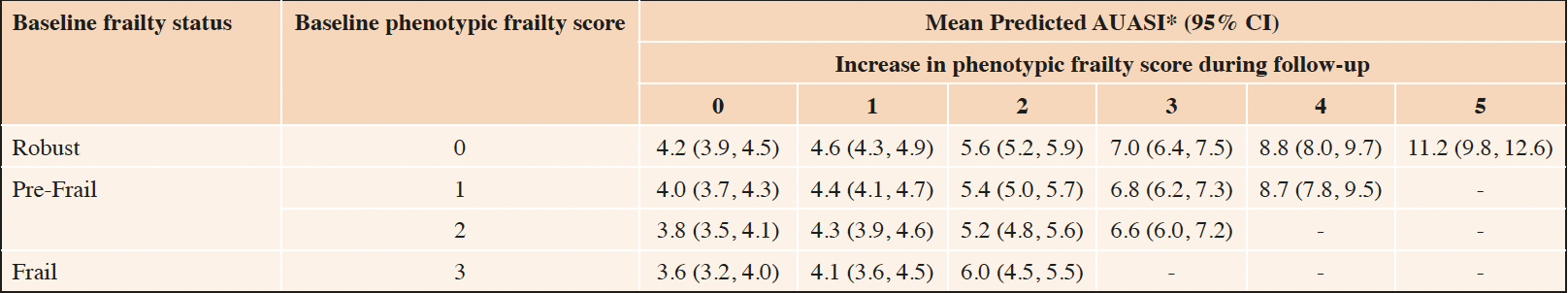
Table 3. Mean predicted AUASI, by cross-classification of baseline and within-person increase in phenotypic frailty score
* Predicted AUASI calculated using linear mixed effects models adjusted for age, site, diabetes, stroke or Parkinson’s disease, chronic obstructive pulmonary disease, angina, heart failure, and hypertension with age set to the median (73 years), and all other covariates set to 0.
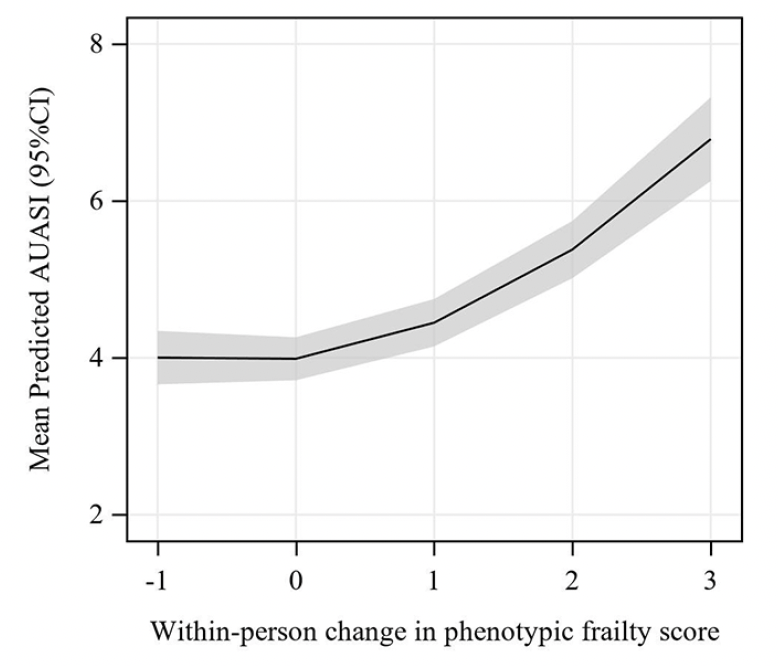
Figure 1. Line plot showing predicted AUASI score by within-person change in phenotypic frailty score
Predicted AUASI calculated using linear mixed effects models adjusted for age, site, diabetes, stroke or Parkinson’s disease, chronic obstructive pulmonary disease, angina, heart failure, and hypertension with age set to the median, baseline frailty phenotype score set to 1, and all other covariates set to 0.
In sensitivity analyses, results were materially unchanged after further adjustment for anxiety/depression, multimorbidity, self-reported general health status, and number of LUTS medications (Supplemental Table 2). Results were also materially unchanged after further adjustment for diuretic medication use and after excluding men with at least weekly urinary incontinence, cognitive impairment, or a baseline phenotypic frailty score of 5 (Supplemental Table 3). When individual frailty phenotype components were examined separately, newly developing each of 4 components (all but shrinking/sarcopenia) was independently associated with worsening LUTS severity (Supplemental Table 4).
Discussion
In this multicenter, prospective cohort study with 7 years of follow-up, we found that older men without clinically meaningful LUTS at baseline who developed increasing phenotypic frailty were also more likely to report greater LUTS severity during follow-up. These non-linear associations were modest in size but consistent across both storage and voiding LUTS, and independent of age, comorbidities, and LUTS treatments. Conversely, baseline frailty status alone was not associated with change in LUTS severity. Importantly, observed associations persisted among men without urinary incontinence or cognitive impairment. Our findings support further investigations into the mechanisms of why frailty and LUTS develop concurrently in order to identify novel frailty-related LUTS phenotypes and treatment targets.
Phenotypic frailty or surrogates of frailty are consistently associated with more severe LUTS in cross-sectional studies. Using data from the same MrOS cohort, our group previously demonstrated that the prevalence of moderate LUTS among men with phenotypic frailty was 46% versus 37% among robust men (adjusted OR= 1.4, 95% CI 1.1, 1.7) and the prevalence of severe LUTS was 13% among men with phenotypic frailty versus 5% among robust men (adjusted OR= 2.5, 95% 1.8, 3.6) (4). Consistent with the current study, these associations were independent of age, comorbidities, or LUTS treatments and persisted among men without urinary incontinence. Similar associations were observed among in small studies among older Korean and Japanese men (35, 36). Our group also previously reported that older men seeking subspecialty treatment for LUTS were more likely to have slow Timed-Up-And-Go-Test times compared to those with other urologic conditions (37) and that slow Timed-Up-And-Go-Test times are associated with detrusor overactivity (38), which can contribute to storage LUTS. This study adds to the cross-sectional literature and is the first study, to our knowledge, to examine the association of baseline phenotypic frailty and change in phenotypic frailty with change in LUTS severity.
Taken together with the cross-sectional studies mentioned above, our study suggests that men with phenotypic frailty and those who develop phenotypic frailty both have greater LUTS severity, but frail men without LUTS at baseline do not have a higher risk of developing worsening LUTS in the future. The conflicting results of models using baseline frailty status versus within-person changes in phenotypic frailty are thought-provoking and highlight several strengths of our approach. First, to determine if worsening phenotypic frailty is an independent risk factor for developing new and progressively worsening LUTS we selected an analytic cohort of older men without moderate-to-severe LUTS, therefore pre-frail and frail men who met our inclusion criteria may be less susceptible to frailty-associated LUTS. Second, coefficient estimates for the association of baseline frailty status with change in a LUTS severity are susceptible to unmeasured confounding. Men who are frail at baseline are almost certainly different than men who are robust at baseline, some but not all of which are captured in the comprehensive questionnaires, interview questions, and functional testing that men agree to complete as MrOS participants. When within-person change in phenotypic frailty is modeled separately from baseline frailty, it is less likely to be biased due to measured or unmeasured baseline characteristics that do not change over time. Adjustment for time-varying confounders, as we have done in this study, further supports the hypothesis that within-person increases in phenotypic frailty are independently associated with worsening LUTS severity. Third, men who have already developed frailty prior to the baseline visit are much more likely to be lost to follow-up due to illness or death, which could bias results towards the null if they developed worsening LUTS prior to being lost to follow-up. Thus, although the effect sizes were modest, these findings suggest that, among men without LUTS at baseline, increasing number of phenotypic frailty components over time is independently associated with concurrent worsening LUTS severity. This study represents an important first step toward understanding whether interventions to prevent or treat frailty could similarly help mitigate age-related LUTS in older men.
Due to an absence of well-validated preclinical models for age-related LUTS beyond traditional models of bladder outlet obstruction, the mechanisms of phenotypic frailty contributing to or developing concurrently with male LUTS remain unknown. Men who develop phenotypic frailty may report that it is “difficult to postpone urination” (urgency) because it takes them longer to travel to the bathroom after they first detect the sensation of a full bladder. Alternatively, men who are developing frailty may be less able to suppress the sensation of urgency via pelvic floor muscle or skeletal abdominal muscle contractions (39). The sensations of “not emptying your bladder completely”, having to “push or strain to begin urination,” or weak urinary stream may be caused by obstruction due to BPH or, alternatively, men with worsening frailty may be unable to generate the same force of urinary expulsion as robust men due to smooth and/or skeletal muscle weakness. Although the role of skeletal muscle in LUTS has been minimally explored, the external urethral sphincter (40), pelvic floor (41), and abdominal musculature (39) are all skeletal muscles suspected to play a role in both micturition control and regulation of bladder sensations. Frail older men produce excess urine at night (42), potentially due to changes in the circadian rhythm of water excretion (43, 44). Lastly, changes within the lower urinary tract that occur with increasing age, such as decreased functional bladder capacity and increased detrusor instability, may be more exaggerated among frail older men (44). Additional work is needed to identify which of these mechanisms contribute to the association between concurrently worsening frailty phenotype and LUTS among older men observed in this study.
The link between phenotypic frailty and LUTS may also be due to a common underlying cause. Although there are several biological mechanisms that contribute to multiple geriatric syndromes, such as white matter intensities (45), our team is particularly interested in the possibility that phenotypic frailty and LUTS are both caused by fundamental biological processes of aging that drive aging-related pathophysiology locally and/or systemically (e.g., the “geroscience hypothesis”). Increasing chronological age is one of the strongest risk factors for LUTS in both men and women (46). In addition to the geriatric syndromes mentioned above that are associated with LUTS, several chronic diseases of aging (47, 48), age-related metabolic (49, 50) and immune (51) derangements, and health-related behaviors that accelerate biological aging (52, 53) are associated with both phenotypic frailty and LUTS. Instead of each chronic disease, phenotype, or syndrome of aging representing a unique LUTS risk factor, perhaps these conditions are all caused by a shared biological mechanism of aging. Although it is impossible to directly measure perceived bladder sensations in animals, evidence from mouse models further supports the aging bladder phenotype as a consequence of centrally mediated adaptive failure (e.g., reduced resilience) due to systemic biologic aging rather than local urogenital tissue changes (54). Future human and animal studies of geroscience mechanisms and interventions should consider including assessments of LUTS and bladder function.
We recognize several limitations to our study. First, MrOS is a cohort of predominantly healthy, older men (to be eligible for the study, men must have been able to walk without assistance and must have lived in the community), most of whom are White. Thus, the results may not be generalizable to younger men or to institutionalized, less-healthy, or more racially diverse men. Second, this is an observational study so men were not randomized to interventions that change their frailty or LUTS status and therefore residual time-varying confounding may explain the observed associations. Third, men with frailty but none/mild LUTS at baseline may have had more severe LUTS in the past and received treatment, which would bias our estimates towards the null when examining the association between frailty status at baseline and change in LUTS. Fourth, the MrOS cohort was initiated more than two decades ago when older generation LUTS treatments that may theoretically contribute to greater risk of frailty, such as non-selective α-blockers, were more commonly prescribed (55). However, we did not observe any evidence of effect modification by LUTS treatment (including medications) and evidence from pooled analyses of randomized clinical trials suggest that older and newer generation α-blockers have similar efficacy. Thus, treatments for LUTS are unlikely to explain observed differences in LUTS severity (56). Lastly, we only tested associations with phenotypic frailty, which is one of multiple valid definitions and conceptual models of frailty (30). It is unknown if other methods to define frailty, such as deficit accumulation, would yield similar results. Similarly, we defined LUTS severity using AUASI total score and subscores, which are commonly used in clinical and urologic research settings, but we did not consider global urinary bother or alternative definitions of LUTS severity, such as number of individual LUTS.
In conclusion, within-person increases in components of phenotypic frailty are associated with concurrent non-linear increases in LUTS severity among older men without clinically meaningful LUTS at baseline. Although observed associations were modest in magnitude, they were independent of age, comorbidities, and LUTS medications and persisted among men without urinary incontinence or cognitive impairment. Further studies are needed to investigate the mechanistic basis of this association and to determine whether frailty interventions could prevent or treat LUTS in older men.
Funding: This work was supported by grants to SRB from the National Institute of Diabetes, Digestive, and Kidney Disorders (grant numbers 1K12DK111028 and U54DK104310) and the National Institute on Aging (grant numbers 1R03AG067937 and 1K76AG074903) and the UCSF Claude D. Pepper Older Americans Independence Center funded by National Institute on Aging (grant number P30 AG044281 to KC). This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR001872. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and the NIH Roadmap for Medical Research (grant numbers U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 TR000128).
Conflict of Interest: The authors have no conflicts.
Author Contributions: S. Bauer: conception and design, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version to be published; C. McCulloch: conception and design, acquisition of data, analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; P. Cawthon: conception and design, acquisition of data, analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; K. Ensrud: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; A. Suskind: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; J. Newman: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; S. Harrison: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; K. Covinsky: conception and design, analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published; L. Marshall: conception and design, acquisition of data, analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published.
Sponsor’s Role: The study funders had no role in the design, methods, subject recruitment, data collections, analysis or preparation of this paper.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in america project: benign prostatic hyperplasia. The Journal of urology. 2008;179(5 Suppl):S75-80. doi:10.1016/j.juro.2008.03.141.
2. Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68(4):804-809. doi:10.1016/j.urology.2006.04.019.
3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37-49. Published 2003/02/01.
4. Bauer SR, Scherzer R, Suskind AM, et al. Co-Occurrence of Lower Urinary Tract Symptoms and Frailty among Community-Dwelling Older Men. Journal of the American Geriatrics Society. 2020. doi:10.1111/jgs.16766.
5. Noguchi N, Chan L, Cumming RG, Blyth FM, Naganathan V. A systematic review of the association between lower urinary tract symptoms and falls, injuries, and fractures in community-dwelling older men. The aging male : the official journal of the International Society for the Study of the Aging Male. 2016;19(3):168-174. doi:10.3109/13685538.2016.1169399.
6. Pesonen JS, Vernooij RWM, Cartwright R, et al. The Impact of Nocturia on Falls and Fractures: A Systematic Review and Meta-Analysis. The Journal of urology. 2019:101097ju0000000000000459. doi:10.1097/ju.0000000000000459.
7. Akerla J, Pesonen JS, Poyhonen A, et al. Impact of lower urinary tract symptoms on mortality: a 21-year follow-up among middle-aged and elderly Finnish men. Prostate cancer and prostatic diseases. 2019;22:317–323. doi:10.1038/s41391-018-0108-z.
8. Welk B, McArthur E, Fraser LA, et al. The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist: a population based cohort study. BMJ (Clinical research ed). 2015;351:h5398. doi:10.1136/bmj.h5398.
9. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of Suicidality and Depression With 5alpha-Reductase Inhibitors. JAMA internal medicine. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089.
10. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA internal medicine. 2019. doi:10.1001/jamainternmed.2019.0677.
11. Agarwal A, Eryuzlu LN, Cartwright R, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. European urology. 2014;65(6):1211-1217. doi:10.1016/j.eururo.2014.01.019.
12. Comprehensive Geriatric Assessment Toolkit for Primary Care Practitioners. British Geriatrics Society. https://www.bgs.org.uk/resources/resource-series/comprehensive-geriatric-assessment-toolkit-for-primary-care-practitioners. Updated 29 January 2019. Accessed 6 October 2021, 2021.
13. Vaughan CP, Markland AD, Smith PP, Burgio KL, Kuchel GA. Report and Research Agenda of the American Geriatrics Society and National Institute on Aging Bedside-to-Bench Conference on Urinary Incontinence in Older Adults: A Translational Research Agenda for a Complex Geriatric Syndrome. Journal of the American Geriatrics Society. 2018;66(4):773-782. doi:10.1111/jgs.15157.
14. Reuben DB, Frank JC, Hirsch SH, McGuigan KA, Maly RC. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. Journal of the American Geriatrics Society. 1999;47(3):269-276. doi:10.1111/j.1532-5415.1999.tb02988.x.
15. Resnick NM. Urinary incontinence. Lancet. 1995;346(8967):94-99. doi:10.1016/s0140-6736(95)92117-6.
16. D’Silva KA, Dahm P, Wong CL. Does this man with lower urinary tract symptoms have bladder outlet obstruction?: The Rational Clinical Examination: a systematic review. JAMA : the journal of the American Medical Association. 2014;312(5):535-542. doi:10.1001/jama.2014.5555.
17. Eckhardt MD, van Venrooij GE, Boon TA. Symptoms and quality of life versus age, prostate volume, and urodynamic parameters in 565 strictly selected men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2001;57(4):695-700. doi:10.1016/s0090-4295(00)01101-8.
18. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569-585. doi:10.1016/j.cct.2005.05.006.
19. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemporary clinical trials. 2005;26(5):557-568. doi:10.1016/j.cct.2005.05.005.
20. Barry MJ, Fowler FJ, Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of urology. 1992;148(5):1549-1557; discussion 1564. Published 1992/11/11.
21. McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. The Journal of urology. 2011;185(5):1793-1803. doi:10.1016/j.juro.2011.01.074.
22. Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? The Journal of urology. 1995;154(5):1770-1774. http://ac.els-cdn.com/S0022534701667806/1-s2.0-S0022534701667806-main.pdf.
23. Barry MJ, Williford WO, Fowler FJ, Jr., Jones KM, Lepor H. Filling and voiding symptoms in the American Urological Association symptom index: the value of their distinction in a Veterans Affairs randomized trial of medical therapy in men with a clinical diagnosis of benign prostatic hyperplasia. The Journal of urology. 2000;164(5):1559-1564. http://ac.els-cdn.com/S0022534705670280/1-s2.0-S0022534705670280-main.pdf.
24. Cauley JA, Cawthon PM, Peters KE, et al. Risk Factors for Hip Fracture in Older Men: The Osteoporotic Fractures in Men Study (MrOS). J Bone Miner Res. 2016;31(10):1810-1819. doi:10.1002/jbmr.2836.
25. Gill SC, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component scale of the 12-item Short-Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Psychiatry research. 2007;152(1):63-71. doi:10.1016/j.psychres.2006.11.005.
26. McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. Journal of clinical epidemiology. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7.
27. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405-411. doi:10.1007/bf01719664.
28. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993;46(2):153-162. http://ac.els-cdn.com/0895435693900534/1-s2.0-0895435693900534-main.pdf.
29. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146-156. Published 2001/03/17.
30. Walston J, Bandeen-Roche K, Buta B, et al. Moving Frailty Toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. Journal of the American Geriatrics Society. 2019;67(8):1559-1564. doi:10.1111/jgs.15928.
31. Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. Journal of the American Geriatrics Society. 2007;55(8):1216-1223. doi:10.1111/j.1532-5415.2007.01259.x.
32. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics. 2nd ed: Springer Science+Business Media, LLC 2012; 2012.
33. Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol. 2016;45(2):565-575. doi:10.1093/ije/dyw040.
34. Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. 2015;36:89-108. doi:10.1146/annurev-publhealth-031914-122559.
35. Jang IY, Lee CK, Jung HW, et al. Urologic symptoms and burden of frailty and geriatric conditions in older men: the Aging Study of PyeongChang Rural Area. Clin Interv Aging. 2018;13:297-304. doi:10.2147/cia.S158717.
36. Soma O, Hatakeyama S, Imai A, et al. Relationship between frailty and lower urinary tract symptoms among community-dwelling adults. Lower urinary tract symptoms. 2020;12(2):128-136. doi:10.1111/luts.12292.
37. Bauer SR, Jin C, Kamal P, Suskind AM. Association Between Lower Urinary Tract Symptoms and Frailty in Older Men Presenting for Urologic Care. Urology. 2020. doi:10.1016/j.urology.2020.09.041.
38. Song S, Jin C, Kamal P, Suskind AM. The association between frailty and detrusor overactivity in older adults. Neurourology and urodynamics. 2020;39(5):1584-1591. doi:10.1002/nau.24414.
39. Neumann P, Gill V. Pelvic floor and abdominal muscle interaction: EMG activity and intra-abdominal pressure. International urogynecology journal and pelvic floor dysfunction. 2002;13(2):125-132. doi:10.1007/s001920200027.
40. Perucchini D, DeLancey JO, Ashton-Miller JA, Peschers U, Kataria T. Age effects on urethral striated muscle. I. Changes in number and diameter of striated muscle fibers in the ventral urethra. American journal of obstetrics and gynecology. 2002;186(3):351-355. doi:10.1067/mob.2002.121089.
41. Vrolijks RO, Notenboom-Nas FJM, de Boer D, et al. Exploring pelvic floor muscle activity in men with lower urinary tract symptoms. Neurourology and urodynamics. 2020;39(2):732-737. doi:10.1002/nau.24267.
42. Monaghan TF, Wagg AS, Bliwise DL, et al. Association between nocturia and frailty among elderly males in a veterans administration population. Aging clinical and experimental research. 2019. doi:10.1007/s40520-019-01416-y.
43. Birder LA, Van Kerrebroeck PEV. Pathophysiological Mechanisms of Nocturia and Nocturnal Polyuria: The Contribution of Cellular Function, the Urinary Bladder Urothelium, and Circadian Rhythm. Urology. 2019;133s:14-23. doi:10.1016/j.urology.2019.07.020.
44. Miller M. Nocturnal polyuria in older people: pathophysiology and clinical implications. Journal of the American Geriatrics Society. 2000;48(10):1321-1329. doi:10.1111/j.1532-5415.2000.tb02608.x.
45. Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(8):818-826. doi:10.1093/gerona/59.8.m818.
46. Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Archives of internal medicine. 2006;166(21):2381-2387. doi:10.1001/archinte.166.21.2381.
47. Lin HJ, Weng SF, Yang CM, Wu MP. Risk of hospitalization for acute cardiovascular events among subjects with lower urinary tract symptoms: a nationwide population-based study. PloS one. 2013;8(6):e66661. doi:10.1371/journal.pone.0066661.
48. Yeniel AO, Ergenoglu AM, Meseri R, et al. Is overactive bladder microvasculature disease a component of systemic atheroscleorosis? Neurourology and urodynamics. 2018;37(4):1372-1379. doi:10.1002/nau.23452.
49. Russo GI, Castelli T, Urzi D, et al. Emerging links between non-neurogenic lower urinary tract symptoms secondary to benign prostatic obstruction, metabolic syndrome and its components: A systematic review. International journal of urology: official journal of the Japanese Urological Association. 2015;22(11):982-990. doi:10.1111/iju.12877.
50. He Q, Wang Z, Liu G, Daneshgari F, MacLennan GT, Gupta S. Metabolic syndrome, inflammation and lower urinary tract symptoms: possible translational links. Prostate cancer and prostatic diseases. 2016;19(1):7-13. doi:10.1038/pcan.2015.43.
51. Siddiqui NY, Helfand BT, Andreev VP, et al. Biomarkers implicated in lower urinary tract symptoms: systematic review and pathway analyses. The Journal of urology. 2019:101097ju0000000000000257. doi:10.1097/ju.0000000000000257.
52. Bradley CS, Erickson BA, Messersmith EE, et al. Evidence of the Impact of Diet, Fluid Intake, Caffeine, Alcohol and Tobacco on Lower Urinary Tract Symptoms: A Systematic Review. The Journal of urology. 2017. doi:10.1016/j.juro.2017.04.097.
53. Mondul AM, Giovannucci E, Platz EA. A Prospective Study of Physical Activity, Sedentary Behavior, and Incidence and Progression of Lower Urinary Tract Symptoms. Journal of general internal medicine. 2020;35(8):2281-2288. doi:10.1007/s11606-020-05814-1.
54. Hardy CC, Keilich SR, Harrison AG, Knight BE, Baker DS, Smith PP. The aging bladder phenotype is not the direct consequence of bladder aging. Neurourology and urodynamics. 2019;38(8):2121-2129. doi:10.1002/nau.24149.
55. Wei JT, Miner MM, Steers WD, et al. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. The Journal of urology. 2011;186(3):971-976. doi:10.1016/j.juro.2011.04.081.
56. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. European urology. 1999;36(1):1-13. doi:10.1159/000019919.
