M. Gagesch1,2, M. Wieczorek1,2, B. Vellas3, R.W. Kressig4, R. Rizzoli5, J. Kanis6,7, W.C. Willett8,9, A. Egli2, W. Lang1,2, E.J. Orav10, H.A. Bischoff-Ferrari1,2,11
1. Department of Aging Medicine, University Hospital Zurich, Zurich, Switzerland; 2. Centre on Aging and Mobility, University Hospital Zurich and University of Zurich, Zurich, Switzerland; 3. Gérontopôle, Toulouse University Hospital, University of Toulouse, UMR INSERM 1295, Toulouse, France; 4. University Department of Geriatric Medicine FELIX PLATTER, Basel, Switzerland; 5. Service of Bone Diseases, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland; 6. Mary McKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia; 7. Centre for Metabolic Bone Diseases, University of Sheffield Medical School, Sheffield, UK; 8. Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, USA; 9. Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, USA; 10. Department of Biostatistics, Harvard T. H. Chan School of Public Health, Boston, USA; 11. University Clinic for Aging Medicine, City Hospital Zurich, Zurich, Switzerland; ClinicalTrials.gov Identifier: NCT01745263
Corresponding Author: Michael Gagesch, MD, Department of Aging Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland, Email: michael.gagesch@usz.ch
J Frailty Aging 2023;12(1)71-77
Published online July 16, 2022, http://dx.doi.org/10.14283/jfa.2022.48
Abstract
Background: The benefits of supplemental vitamin D3, marine omega-3 fatty acids, and a simple home exercise program (SHEP) on frailty prevention in generally healthy community-dwelling older adults are unclear.
Objective: To test the effect of vitamin D3, omega-3s, and a SHEP, alone or in combination on incident pre-frailty and frailty in robust older adults over a follow-up of 36 months.
Methods: DO-HEALTH is a multi-center, double-blind, placebo-controlled, 2x2x2 factorial randomized clinical trial among generally healthy European adults aged 70 years or older, who had no major health events in the 5 years prior to enrollment, sufficient mobility and intact cognitive function. As a secondary outcome of the DO-HEALTH trial, among the subset of participants who were robust at baseline, we tested the individual and combined benefits of supplemental 2,000 IU/day of vitamin D3, 1 g/day of marine omega-3s, and a SHEP on the odds of being pre-frail and frail over 3 years of follow-up.
Results: At baseline, 1,137 out of 2,157 participants were robust (mean age 74.3 years, 56.5% women, mean gait speed 1.18 m/s). Over a median follow-up time of 2.9 years, 696 (61.2%) became pre-frail and 29 (2.6%) frail. Odds ratios for becoming pre-frail were not significantly lower for vitamin D3, or omega 3-s, or SHEP, individually, compared to control (placebo for the supplements and control exercise). However, the three treatments combined showed significantly decreased odds (OR 0.61 [95% CI 0.38-0.98; p=0.04) of becoming pre-frail compared to control. None of the individual treatments or their combination significantly reduced the odds of becoming frail.
Conclusion: Robust, generally healthy and active older adults without major comorbidities, may benefit from a combination of high-dose, supplemental vitamin D3, marine omega-3s, and SHEP with regard to the risk of becoming pre-frail over 3 years.
Key words: Frailty prevention, clinical trial, older adults.
Introduction
Physical frailty is an age-related medical syndrome affecting the health of older adults, leading to an increased susceptibility of the individual against multiple negative health outcomes (1, 2). With the ongoing growth of the global aging population, the impact of frailty on the individual and also on the health economic level is expected to increase substantially (3). Of note, frailty is a dynamic process and is potentially reversible if detected early (4, 5). While existing evidence suggests a noteworthy role for vitamin D, omega-3s and physical activity on the progression of frailty, the combination of low-cost interventions including the supplementation of key nutrients and increased exercise might appear favorable also for the primary prevention of frailty (6, 7).
Low 25-hydroxyvitamin D (Calcidiol) levels have been associated with frailty (8, 9) and pre-frailty (10) in several observational studies of community-dwelling adults. Mechanistically, it has been suggested that vitamin D supplementation may reduce frailty via several pathways, including positive effects on muscle and bone health (11, 12). Consequently, Vitamin D supplementation might play an important role in the prevention of frailty and the at-risk state of pre-frailty. However, evidence from the current literature is scarce and results from randomized controlled trials are still missing (13, 14).
Omega 3-fatty acids have been linked to skeletal muscle health (6). Mechanistically, it has been suggested that omega-3s reduce pro-inflammatory cytokines, improve insulin sensitivity, stimulate muscle protein synthesis via the mTOR signaling pathway, and reduce reactive oxygen species (ROS) in mitochondria (6, 15-18). Although evidence so far appears limited, omega-3 supplementation may provide relevant benefits on muscle function in older adults (19).
Low physical activity is a key component of physical frailty (20). Mechanistically, low physical activity has been associated with inflammaging and impaired mitochondrial function leading to reduced oxidative capacity linked to the development of sarcopenia and frailty (21-23).
Prior results from observational studies suggested that slowness, low activity level and weakness separate frail and non-frail groups already 6 years prior to clinically overt onset of frailty (RR ranging from 1.39 – 1.94) (24). These findings indicate the importance of low physical performance on the trajectory towards frailty. Further, the combination of supplemental vitamin D and physical exercise might provide an additive effect on muscle function in older adults (25).
In regard to health and active aging, preventing pre-frailty among robust older adults appears important as pre-frail individuals are already at risk of adverse outcomes including falls, disability, hospitalizations and premature mortality (2). The DO-HEALTH study of generally healthy older European adults reported that 43% of their participants at baseline were considered pre-frail (26). Therefore, it is critical to timely identify potential interventions to address pre-frailty for the prevention of frailty and its associated negative health outcomes in older adults (27).
Randomized trials assessing the treatment of vitamin D3, omega-3s supplementation, and home exercise, alone or in combination, for the primary prevention of pre-frailty and frailty are missing to date (28). Therefore, we aim to test the individual and combined effects of treatment with vitamin D3, omega-3s, and a simple home exercise program (SHEP) for the prevention of pre-frailty and frailty in generally healthy and robust adults age 70 and older from the large DO-HEALTH trial.
Methods
Trial design and oversight
DO-HEALTH is a three–year, multi-center, double-blind, 2x2x2 factorial design randomized controlled clinical trial (NCT01745263) designed to support healthy aging in European adults age 70 and older (29, 30). Clinical visits were at baseline, 1, 2, and 3 years, with intermediate phone calls every three months. The detailed trial design and protocol have been described elsewhere (29, 30). The 2,157 trial participants were generally healthy, community-dwelling adults aged 70 years and older, recruited from seven centers in five European countries: Zurich, Basel, Geneva (Switzerland); Berlin (Germany); Innsbruck (Austria); Toulouse (France); and Coimbra (Portugal). Inclusion criteria were absence of major health events in the five years prior to enrolment including cancer and cardiovascular events, sufficient mobility to come to the study centers, and intact cognitive function with a MMSE score of at least 24 points. All participants signed informed consent and the study protocol was approved by ethical and regulatory agencies of all five countries.
Interventions
The three interventions were randomly assigned in eight treatment groups according to the 2x2x2 factorial trial design: 2000 IU/d of vitamin D3,1 g/d of omega-3s and SHEP (n = 264); vitamin D3 and marine omega-3s (n = 265); vitamin D3 and SHEP (n = 275); vitamin D3 alone (n = 272); omega-3s and SHEP (n = 275); omega-3s alone (n = 269); SHEP alone (n = 267); or placebo (n = 270). Each participant received two study capsules per day: each active vitamin D capsule contained 1000 IU of Vitamin D3 stabilized with dl-α-tocopherol (vitamin E, 2.5 pro mill); each active omega-3s capsule contained 500 mg of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in a ratio of 1:2; and each placebo capsule contained high oleic sunflower oil. The SHEP was an unsupervised strength-training exercise of 30 minutes, three times per week compared with an attention control exercise program focused on joint flexibility of 30 minutes three times a week (details of the program are presented in Supplementary Table 1). All participants were required to limit the use of vitamin D from all other supplemental sources, including multivitamins, to the recommended dietary allowance intake for older adults (800 IU per day), and to forego any supplemental omega-3 intake.30 In addition, participants were provided with a personal diary to record their adherence to the study interventions and encouraged to implement an exercise routine in order to allow optimal adherence to the SHEP. Study personnel collected the diary and documented the routine at each clinical visit. Details on adverse events and adherence have been reported earlier (29).
Operationalization of pre-frailty and frailty
Frailty status was assessed at baseline and annually over three years of follow-up according to the five domains of the Fried physical frailty phenotype (20), with limited adaptions to the five domains to fit the variables available from the DO-HEALTH dataset. For weakness, grip strength was recorded in Kilopascal (kPa) from the best of three consecutive trials using the dominant hand with a Martin Vigorimeter (KLS Martin Group, Tuttlingen, Germany). We used cut points determined by the lowest quintile approach as did Fried and colleagues in their landmark study, stratified by sex and age group (<75 years and ≥75 years) (31). Fatigue was defined by a positive answer to the self-reported question: “In the last month, have you had too little energy to do things you wanted to?” from the Survey of Health, Ageing and Retirement in Europe (SHARE) Frailty Instrument (SHARE-FI) (32). Involuntary relevant weight loss was defined as >4.5kg or a >5% change in weight in 1 year at follow-up. Low gait speed was defined as ≤0.65m/s (men ≤173cm, women ≤159cm) and ≤0.76 (men >173cm, women >159cm) from the Short Physical Performance Battery (SPPB) (33). Finally, low activity level was defined as a response of “Less than once a week» to the self-report question: “How often do you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or going on a walk?” from SHARE-FI (32). Participants with zero positive items were classified as robust, and participants with one or two positive items were classified as being pre-frail, while participants with three or more positive items were classified as frail according to the definition by Fried et al (20). For the present study, robust participants at baseline who became pre-frail at any time point over the follow-up but who did not become frail were considered as pre-frail. Participants who were robust at baseline and became frail at any time point over the follow-up were considered as frail.
Assessment of biomarkers
As described previously, DSM Nutritional Products R&D Analytics performed 25-hydroxyvitamin D measurements and the Research Toxicology Center performed polyunsaturated fatty acid measurements (EPA and DHA) by sensitive and selective assays based on liquid chromatography coupled to a mass spectrometry detection system at baseline and at 12, 24, and 36 months (29). Mass spectrometry detection systems were monitored with standard, quality control, and human National Institute of Standards and Technology plasma reference samples. In accordance with the DO-HEALTH main paper, vitamin D repleteness was defined as serum 25-hydroxyvitamin D levels ≥20 ng/mL, vitamin D deficiency as serum 25-hydroxyvitamin D levels <20 ng/mL and severe deficiency as serum 25-hydroxyvitamin D levels <12 ng/mL (29).
Statistical analysis
The analytic dataset was a subset of DO-HEALTH participants who were identified as robust at baseline (n=1137). Baseline characteristics of the study population are described overall and by treatment group. Normally distributed continuous variables are presented as means and standard deviations (SD) and non-normal variables as median and inter quartile range (IQR). Categorical variables are presented in frequencies and percentages. Differences between treated and non-treated participants at baseline were tested using the Wilcoxon rank sum test, t-test or chi-square test, for non-normal, normal and categorical variables, respectively.
With frailty status being coded as robust/pre-frail/frail, and in violation of the proportional odds assumption (p=0.004), we fit a multinomial logistic regression model comparing 1) participants who became pre-frail at any time point over the three-year follow-up vs. participants who remained robust over three years; and 2) participants who became frail at any time point over the three-year follow-up vs. participants who remained robust over three years.
We first tested the interaction effect of the treatment groups. Neither the three-way treatment interaction of vitamin D3*omega-3s*SHEP (p=0.998) nor the three two-way interaction effects of vitamin D3*omega-3s (p=0.27), vitamin D*SHEP (p=0.34), and omega-3s*SHEP (p=0.38) were significant in the multinominal logistic regression model. Therefore, the main effects of treatments vitamin D3, omega-3s, and SHEP were included in the models.
To test the individual and combined effects of study treatments, we adjusted for the following randomization stratification variables: age, sex, low-trauma falls (yes/no) during the 12 months preceding the randomization day and study site.
Results are presented as odds ratios and 95% confidence intervals. The type I error rate was set at 5%. All statistical analyses were performed using SAS v9.4 (SAS Institute, Inc., Cary, NC, United States).
Results
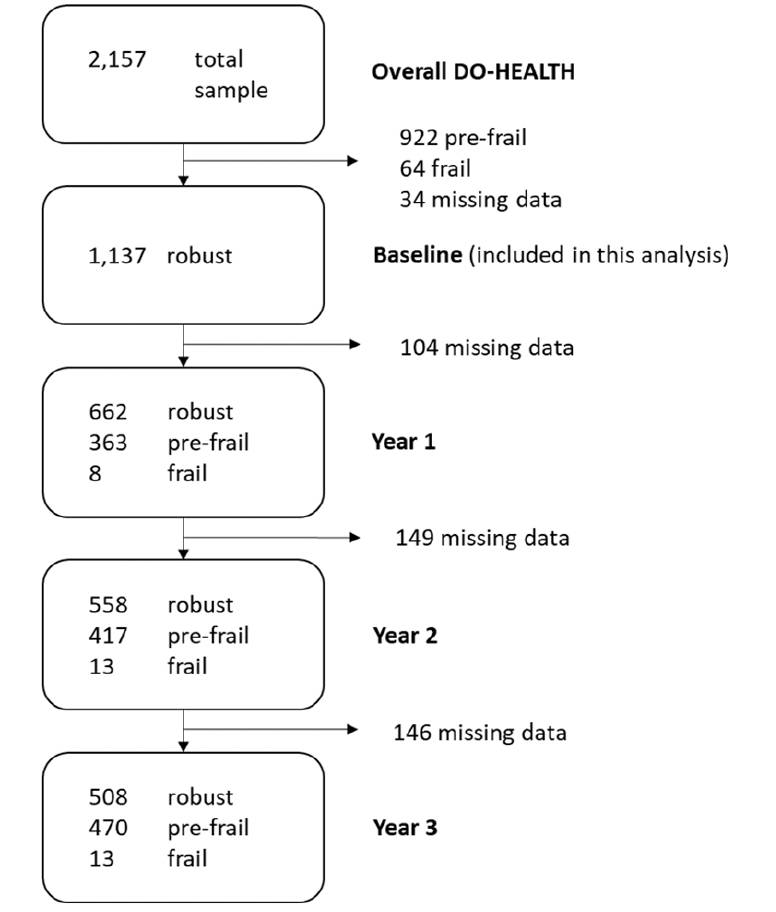
Of the 2,157 DO-HEALTH participants, 1,137 were robust at baseline and were included in this analysis. Of those, 56.5% were women, mean age was 74.3 (±4.0) years, mean BMI was 26.1 kg/m2 (±4.0) and 432 (38%) were living alone. Participants reported a mean physical activity volume of 40.3 (±32.9) metabolic equivalent of task-hours (MET-hrs) per week and a mean physical performance score of 11.3 points (±1.1) on the SPPB (score range: min. 0; max. 12 points). Upon enrolment, mean serum 25-hydroxyvitamin D concentration was 23.0 ng/mL while 38.0% of participants were vitamin D deficient with 25-hydroxyvitamin D levels below 20 ng/mL. In all, baseline characteristics of the treatment and non-treatment groups were balanced. Table 1 summarizes the baseline characteristics of the included participants, overall and by treatment group. With regard to adherence, 75.2% of participants took at least 80% of their total study pills and 54.5% of participants performed their exercise program at least two times per week.
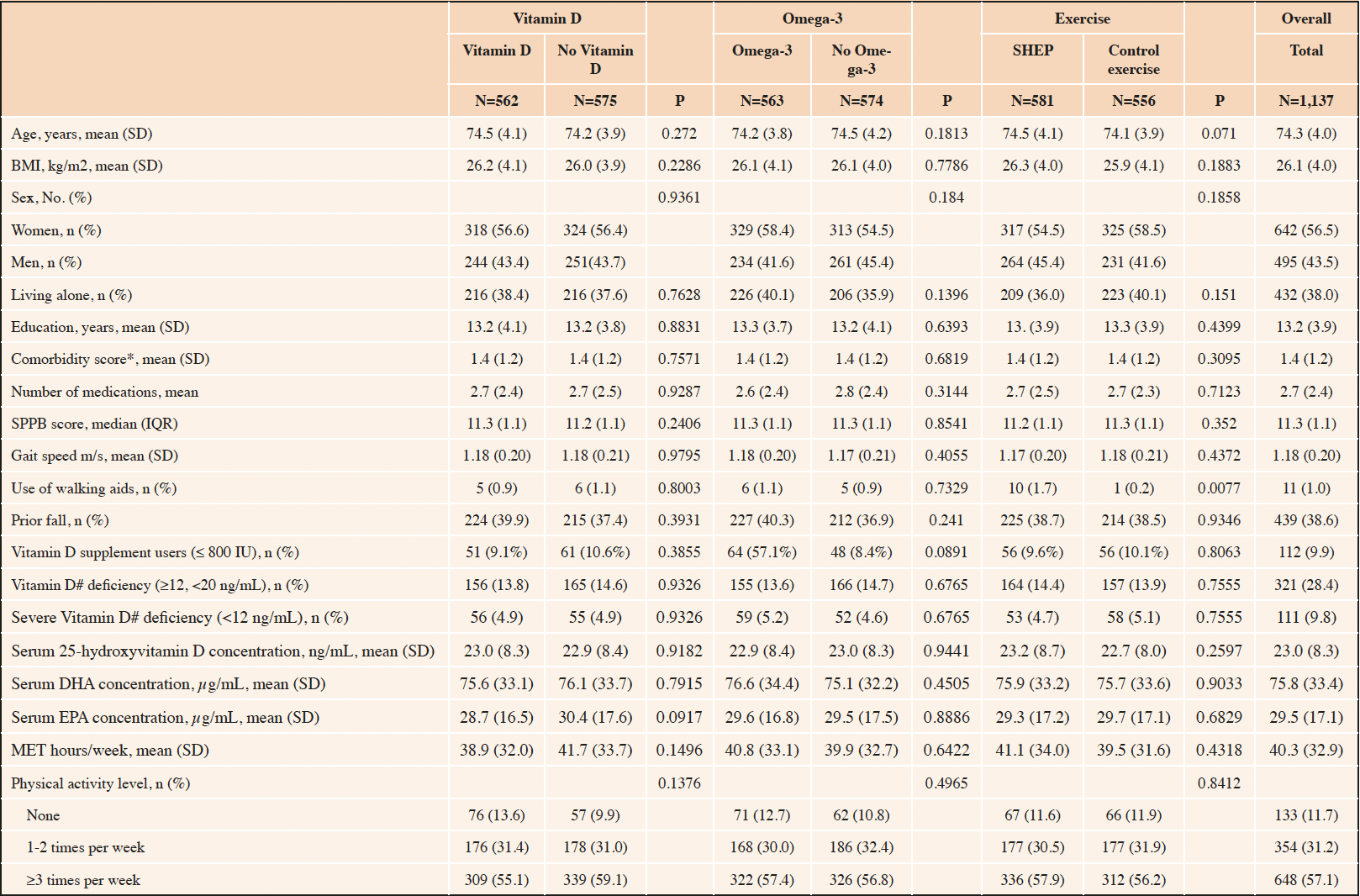
Table 1. Baseline characteristics of the DO-HEALTH trial participants that were robust at baseline and received individual treatments (n=1,137)
Legend: SD (standard deviation), BMI (Body Mass Index), *Comorbidity score by self-administered questionnaire51, #25-hydroxyvitamin D concentrations; SPPB (Short physical performance battery), IQR (interquartile range), DHA (Docosahexaenoic acid), EPA (Eicosapentaenoic acid); MET (metabolic equivalent of task)
Odds of becoming pre-frail by treatment group
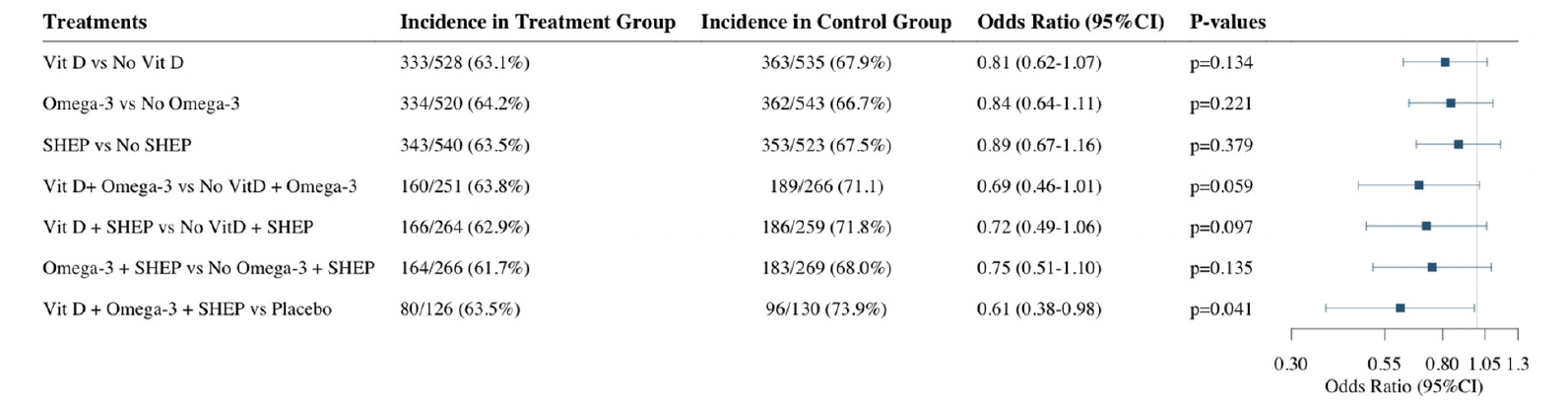
Of the 1,137 robust participants at baseline, 691 (61.2%) became pre-frail over the three-year follow-up (Figure 1). Adjusting for age, sex, prior falls, and study site, the odds ratios (OR, 95% CI) of becoming pre-frail over the 3 year follow-up comparing the individual treatment effects were 0.81 (0.62-1.07; p=0.13) for receiving vitamin D3 vs. no vitamin D3, 0.84 (0.64-1.11; p=0.22) for receiving omega-3s vs. no omega-3s, and 0.89 (0.67-1.16; p=0.38) for receiving SHEP vs. no SHEP. In regard to the combined treatment effects, the odds ratios (OR, 95% CI) of becoming pre-frail over the 3 year follow-up were 0.69 (0.46-1.01; p=0.06) for receiving vitamin D3 plus omega-3s vs. no vitamin D3 and omega-3s, 0.75 (0.51-1.10; p=0.14) for receiving omega-3s plus SHEP vs. no omega-3s and SHEP, 0.72 (0.49-1.06; p=0.10) for receiving vitamin D3 plus SHEP vs. no vitamin D3 and SHEP, and 0.61 (0.38-0.98; p=0.04) for receiving all 3 treatments combined vs. placebo (Figure 2).
For this analysis we included only participants considered robust at baseline from the total DO-HEALTH study population according to the applied frailty phenotype
Vit D = Vitamin D supplementation, Omega-3 = Omega-3 fatty acids supplementation, SHEP = simple home exercise program
Odds of becoming frail by treatment group
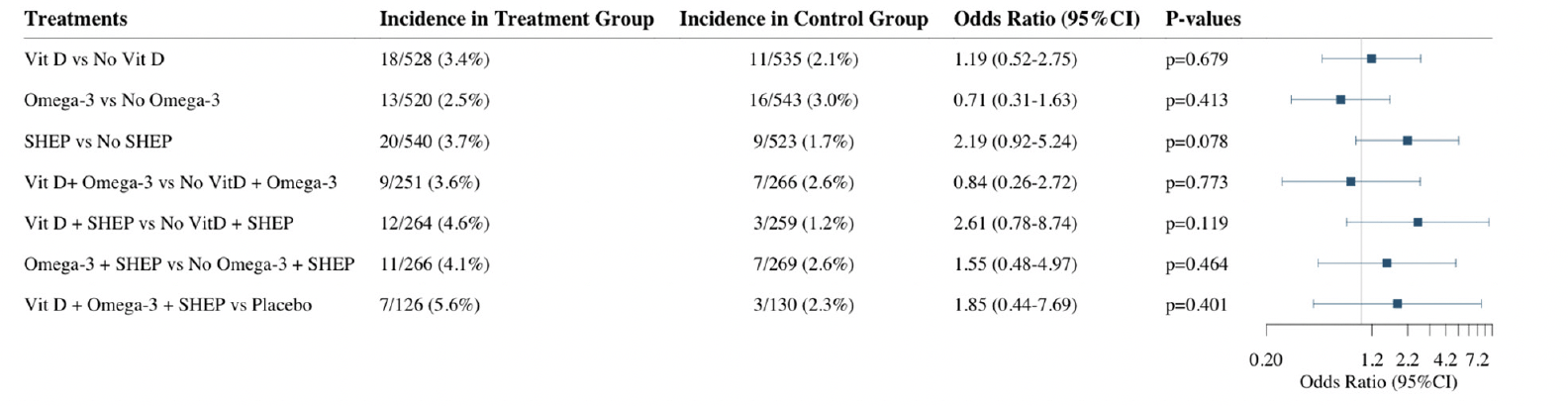
Over the three-year follow-up, 29 (2.6%) participants who were robust at baseline became frail. Given the small incidence of frailty in this generally healthy study population, there was limited power to test treatment effects for this outcome. Adjusting for age, sex, prior falls, and study site, the odds ratios (OR, 95% CI) of becoming frail over the three-year follow-up comparing the individual treatment effects were 1.19 (0.52-2.75; p=0.68) for receiving vitamin D3 vs. no vitamin D3, 0.71 (0.31-1.63; p=0.41), for receiving omega-3s vs. no omega-3s, and 2.19 (0.92-5.24; p=0.08) for receiving SHEP vs. no SHEP. In regard to the combined treatment effects, the odds ratios (OR, 95% CI) of becoming frail over the 3 year follow-up were 0.84 (0.26-2.72; p=0.77) for receiving vitamin D3 plus omega-3s vs. no vitamin D3 and omega-3s, 1.54 (0.48-4.95; p=0.46) for receiving omega-3s plus SHEP vs. no omega-3s and SHEP, 2.61 (0.78-8.74; p=0.12) for receiving vitamin D3 plus SHEP vs. no vitamin D3 and SHEP, and 1.85 (0.44-7.69; p=0.40) for receiving the 3 treatments compared to control (placebo for the supplements and control exercise) (Figure 3).
Vit D = Vitamin D supplementation, Omega-3 = Omega-3 fatty acids supplementation, SHEP = simple home exercise program
Discussion
In this three-year, double-blind, randomized controlled trial among 1,137 older adults who were robust at baseline, the combined interventions of daily supplemental 2000 IU vitamin D3 plus daily 1g marine omega-3 plus SHEP had significant benefits with regard to the prevention of pre-frailty. However, this benefit was not significant for the 3 treatments individually, highlighting the importance of the additive effect of all three preventive strategies.
Given that the DO-HEALTH participants were generally healthy, physically active and robust, only few (2.6%) developed frailty over the 3-year follow-up. Thus, we were underpowered to address the odds of frailty by treatment group, and were not able to detect beneficial treatment effects extending from pre-frailty to frailty.
The association of vitamin D and frailty status has been investigated in multiple observational studies, demonstrating that low vitamin D levels are associated with increased risk of becoming frail (34). However, to the best of our knowledge, no prior randomized clinical trial in generally healthy and largely vitamin D replete community dwelling older adults has investigated the effect of vitamin D3 supplementation for the prevention of pre-frailty as a predefined primary outcome. Therefore, DO-HEALTH contributes important data to the existing literature, exhibiting a beneficial effect of 2,000 IU vitamin D3 supplementation on the odds of becoming pre-frail when combined with omega-3 and SHEP.
For omega-3s, to our knowledge, no randomized controlled trials have investigated the effect of omega-3 supplementation on the development of pre-frailty as a primary outcome. In an analysis of participants in the InCHIANTI study, higher physical function was associated with higher levels of n-3 PUFAs (35). The Multidomain Alzheimer Preventive Trial (MAPT), including over 1,500 community-dwelling older adults investigating omega-3s supplementation and a multi-domain intervention (physical activity, cognitive training, and nutritional advice), alone or in combination, compared with placebo, did not find a positive signal in regard to the development of frailty by supplemental omega-3s over a 3 year period (36). However, this trial did not report on the prevention of the at risk-state of pre-frailty and included participants with memory complaints and functional impairments. Therefore, we are limited in our ability to make direct comparisons to other study populations of the potentially beneficial effect of omega-3s combined with the other treatments to the generally healthy DO-HEALTH population.
In regard to exercise interventions, systematic reviews investigating the impact of exercise in non-frail, pre-frail and frail community dwelling older adults already suggested its beneficial effect on frailty progression. However, there still remains some uncertainty regarding the optimal duration, frequency, and type of exercise (28, 37, 38). In a recent randomized controlled trial among 100 frail participants, a multicomponent exercise program (including proprioception, aerobic, strength, and stretching exercises for 65 minutes, 5 days per week, for 24 weeks) led to a reduction in frailty level of 30% in the intervention group (39). In our study, the unsupervised SHEP program of DO-HEALTH for 30 minutes 3 times per week for 3 years had no significant effect on the incidence of pre-frailty and frailty individually, though it significantly reduced the odds of becoming pre-frail when combined with the other treatments.
The combined effect of the three DO-HEALTH interventions on the prevention of pre-frailty may be explained by their complementary influences on the complex pathophysiological pathways that are linked to the development of frailty, including muscle function, chronic inflammation, cardiovascular health and immune system regulations even among robust, physically active and generally healthy older adults (6, 40, 41). Therefore, our findings of a combined effect might be explained by additive ergogenic properties of the three interventions (42) and their potential effects on the reduction of chronic low-grade inflammation (43-45). In particular, vitamin D has been described as independently and inversely associated with interleukin (6), suggesting a potential anti-inflammatory role in older individuals (46). Further, anti-inflammatory properties have also been described for omega-3s (6). Besides their role in the prevention of cardiovascular diseases, it is suggested they alter the lipid profile, producing an anti-inflammatory effect by improving vascular endothelial function and insulin sensitivity (47). In addition to the well-established influence of vitamin D on musculoskeletal health (48), mechanistic studies also support the involvement of vitamin D in the development of cardiovascular diseases (49). Lastly, besides the known effects of exercise on cardiovascular health, its anti-inflammatory effects might be mediated by mechanisms on cytokine regulation including interleukin 6, especially in older adults (44, 50).
Our study has several strengths. Primarily, this is the first multicenter randomized clinical trial investigating the individual and combined effects of supplemental vitamin D3, omega-3s and a SHEP on the primary prevention of pre-frailty in a large sample of generally healthy and community dwelling European older adults over a follow-up of 3 years. In addition, frailty status was a predefined secondary outcome in the study protocol, captured by a standardized procedure, and assessed by trained study personal according to a strict protocol at all sites.
However, a few limitations can be acknowledged. First, this is a secondary analysis of the DO-HEALTH trial, designed to investigate the effect of the three interventions in regard to 6 primary outcomes (change in systolic and diastolic blood pressure, physical performance, cognitive function, and incidence rates of non-vertebral fractures and infections over 3 years). In addition, the relatively good health and low incidence of frailty, despite the DO-HEALTH inclusion criteria containing prior falls, resulted in the analysis of overt frailty as a primary outcome to be underpowered. Furthermore, supplemental vitamin D3 800 IU/d was allowed in all participants, and SHEP was randomized against control exercise (joint flexibility 3x 30 min/wk.).
Overall, our results suggest a beneficial effect of supplemental vitamin D3, omega-3s and an unsupervised SHEP in combination for the prevention of pre-frailty in generally healthy older adults. Further investigation is warranted to determine whether the combined benefits of the DO-HEALTH interventions are superior compared to a healthy and active lifestyle including a comparative amount of dietary omega-3s and regularly physical exercise in this target group. At the same time, the potential benefits of the combined daily supplementation of 2,000 IU of vitamin D3, 1g of marine omega-3s and a SHEP in regard to the prevention of pre-frailty and frailty should be further investigated in additional clinical trials including the in-depth study of the complex underlying mechanisms on the molecular level.
Funding/Support: The DO-HEALTH study was funded by the Seventh Framework Program of the European Commission (grant agreement 278588), the University of Zurich (Chair for Geriatric Medicine and Aging Research), DSM Nutritional Products, Roche, NESTEC, Pfizer, and Streuli. Open Access funding provided by University Zurich.
Role of the Funder/Sponsor: The funding/supporting organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Acknowledgements: We thank the members of the DO-HEALTH Research Group, all study personnel at the seven study sites and all participants for their involvement in and commitment to this study. We do thank Dr. Erin West for her critical language editing.
Conflicts of Interest: The authors declare no conflict of interest in regard to this work.
Ethical standards: The DO-HEALTH study was approved by ethics and regulatory agencies of all 5 countries and the study protocol has been previously published (30). A data and safety monitoring board oversaw the study.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. Jun 2013;14(6):392-7. doi:10.1016/j.jamda.2013.03.022
2. Vermeiren S, Vella-Azzopardi R, Beckwee D, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc. Dec 01 2016;17(12):1163.e1-1163.e17. doi:10.1016/j.jamda.2016.09.010
3. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. The Lancet. 2019;394(10206):1365-1375. doi:10.1016/S0140-6736(19)31786-6
4. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. Feb 27 2006;166(4):418-23. doi:10.1001/archinte.166.4.418
5. Negm AM, Kennedy CC, Thabane L, et al. Management of Frailty: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. Journal of the American Medical Directors Association. 2019;20(10):1190-1198. doi:10.1016/j.jamda.2019.08.009
6. Tessier AJ, Chevalier S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients. Aug 16 2018;10(8)doi:10.3390/nu10081099
7. Oliveira JS, Pinheiro MB, Fairhall N, et al. Evidence on Physical Activity and the Prevention of Frailty and Sarcopenia Among Older People: A Systematic Review to Inform the World Health Organization Physical Activity Guidelines. Journal of physical activity & health. Aug 11 2020:1-12. doi:10.1123/jpah.2020-0323
8. Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61doi:10.1093/gerona/61.6.589
9. Pabst G, Zimmermann AK, Huth C, et al. Association of low 25-hydroxyvitamin D levels with the frailty syndrome in an aged population: results from the KORA-age Augsburg study. J Nutr Health Aging. Mar 2015;19(3):258-64. doi:10.1007/s12603-014-0546-9
10. Vogt S, Decke S, de Las Heras Gala T, et al. Prospective association of vitamin D with frailty status and all-cause mortality in older adults: Results from the KORA-Age Study. Preventive medicine. Apr 2015;73:40-6. doi:10.1016/j.ypmed.2015.01.010
11. Artaza-Artabe I, Saez-Lopez P, Sanchez-Hernandez N, Fernandez-Gutierrez N, Malafarina V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas. Apr 14 2016;doi:10.1016/j.maturitas.2016.04.009
12. Clegg A, Hassan-Smith Z. Frailty and the endocrine system. The lancet Diabetes & endocrinology. Jul 12 2018;doi:10.1016/s2213-8587(18)30110-4
13. Hernandez Morante JJ, Gomez Martinez C, Morillas-Ruiz JM. Dietary Factors Associated with Frailty in Old Adults: A Review of Nutritional Interventions to Prevent Frailty Development. Nutrients. Jan 5 2019;11(1)doi:10.3390/nu11010102
14. Bolzetta F, Stubbs B, Noale M, et al. Low-dose vitamin D supplementation and incident frailty in older people: An eight year longitudinal study. Exp Gerontol. Jan 2018;101:1-6. doi:10.1016/j.exger.2017.11.007
15. Liu CK, Lyass A, Larson MG, et al. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age (Dordr). Feb 2016;38(1):1. doi:10.1007/s11357-015-9864-z
16. Perez-Tasigchana RF, Leon-Munoz LM, Lopez-Garcia E, et al. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing. Sep 1 2017;46(5):807-812. doi:10.1093/ageing/afx023
17. El Assar M, Angulo J, Rodriguez-Manas L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free radical biology & medicine. Aug 15 2019;doi:10.1016/j.freeradbiomed.2019.08.011
18. Murton AJ. Muscle protein turnover in the elderly and its potential contribution to the development of sarcopenia. Proc Nutr Soc. Nov 2015;74(4):387-96. doi:10.1017/S0029665115000130
19. Huang YH, Chiu WC, Hsu YP, Lo YL, Wang YH. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients. Dec 4 2020;12(12)doi:10.3390/nu12123739
20. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. Mar 2001;56(3):M146-56.
21. Adelnia F, Urbanek J, Osawa Y, et al. Moderate-to-Vigorous Physical Activity Is Associated With Higher Muscle Oxidative Capacity in Older Adults. Journal of the American Geriatrics Society. 2019;67(8):1695-1699. doi:https://doi.org/10.1111/jgs.15991
22. Gomes M, Figueiredo D, Teixeira L, et al. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing. Jan 20 2017;46(1):71-77. doi:10.1093/ageing/afw165
23. Izquierdo M, Merchant RA, Morley JE, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. The journal of nutrition, health & aging. 2021/07/01 2021;25(7):824-853. doi:10.1007/s12603-021-1665-8
24. Stenholm S, Ferrucci L, Vahtera J, et al. Natural Course of Frailty Components in People Who Develop Frailty Syndrome: Evidence From Two Cohort Studies. J Gerontol A Biol Sci Med Sci. Aug 1 2018;doi:10.1093/gerona/gly132
25. Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D(3) supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ open. Jul 20 2017;7(7):e014619. doi:10.1136/bmjopen-2016-014619
26. Gagesch M, Chocano-Bedoya PO, Abderhalden LA, et al. Prevalence of Physical Frailty: Results from the DO-HEALTH Study. The Journal of Frailty & Aging. 2021/05/03 2021;doi:10.14283/jfa.2021.18
27. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. The Lancet. 2019;394(10206):1376-1386. doi:10.1016/S0140-6736(19)31785-4
28. Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI database of systematic reviews and implementation reports. Jan 2018;16(1):140-232. doi:10.11124/jbisrir-2017-003382
29. Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA. 2020;324(18):1855-1868. doi:10.1001/jama.2020.16909 %J JAMA
30. Bischoff-Ferrari HA, de Godoi Rezende Costa Molino C, Rival S, et al. DO-HEALTH: Vitamin D3 – Omega-3 – Home exercise – Healthy aging and longevity trial – Design of a multinational clinical trial on healthy aging among European seniors. Contemporary clinical trials. 2021/01/01/ 2021;100:106124. doi:https://doi.org/10.1016/j.cct.2020.106124
31. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age and Ageing. July 1, 2011 2011;40(4):423-429. doi:10.1093/ageing/afr051
32. Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. Jun 2009;64(6):675-81. doi:10.1093/gerona/glp012
33. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. Mar 1994;49(2):M85-94.
34. Marcos-Pérez D, Sánchez-Flores M, Proietti S, et al. Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2020;12(8):2286. doi:10.3390/nu12082286
35. Abbatecola AM, Cherubini A, Guralnik JM, et al. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. Feb 2009;12(1):25-32. doi:10.1089/rej.2008.0799
36. Guerville F, de Souto Barreto P, Giudici KV, Rolland Y, Vellas B. Association of 3-Year Multidomain Intervention and Omega-3 Supplementation with Frailty Incidence. J Am Geriatr Soc. Aug 2019;67(8):1700-1706. doi:10.1111/jgs.15994
37. de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC geriatrics. Dec 2 2015;15:154. doi:10.1186/s12877-015-0155-4
38. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. The British journal of general practice : the journal of the Royal College of General Practitioners. Jan 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
39. Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, et al. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J Am Med Dir Assoc. May 1 2016;17(5):426-33. doi:10.1016/j.jamda.2016.01.019
40. Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. BioMed research international. 2015;2015:953241. doi:10.1155/2015/953241
41. Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. Oct 2009;64(10):1049-57. doi:10.1093/gerona/glp076
42. Dalle S, Rossmeislova L, Koppo K. The Role of Inflammation in Age-Related Sarcopenia. Review. Frontiers in physiology. 2017-December-12 2017;8doi:10.3389/fphys.2017.01045
43. Ticinesi A, Meschi T, Lauretani F, et al. Nutrition and Inflammation in Older Individuals: Focus on Vitamin D, n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients. Mar 29 2016;8(4):186. doi:10.3390/nu8040186
44. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. Sep 2011;11(9):607-15. doi:10.1038/nri3041
45. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology. 2018/09/01 2018;15(9):505-522. doi:10.1038/s41569-018-0064-2
46. De Vita F, Lauretani F, Bauer J, et al. Relationship between vitamin D and inflammatory markers in older individuals. AGE. 2014/08/03 2014;36(4):9694. doi:10.1007/s11357-014-9694-4
47. Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. The Cochrane database of systematic reviews. Feb 29 2020;3(3):Cd003177. doi:10.1002/14651858.CD003177.pub5
48. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. Dec 2004;80(6 Suppl):1678s-88s. doi:10.1093/ajcn/80.6.1678S
49. Cosentino N, Campodonico J, Milazzo V, et al. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients. Oct 14 2021;13(10)doi:10.3390/nu13103603
50. Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. Feb 20 2001;103(7):947-53.
51. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis and rheumatism. Apr 15 2003;49(2):156-63. doi:10.1002/art.10993