A. Ophey1,*, T. Brijoux2,*, A. Conrad1, A.-K. Folkerts1, S. Zank2,3, E. Kalbe1
1. Department of Medical Psychology | Neuropsychology & Gender Studies, Center for Neuropsychological Diagnostics and Intervention (CeNDI), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; 2. University of Cologne, Cologne Center for Ethics, Rights, Economics, and Social Sciences of Health, Germany;
3. University of Cologne, Faculty of Human Sciences, Rehabilitative Gerontology, Germany; *contributed equally
Corresponding Author: Dr. Anja Ophey, Kerpener Str. 68, 50937 Cologne, Germany, phone +49 221 478-32976, fax +49 221 478-3420, anja.ophey@uk-koeln.de
J Frailty Aging 2023;12(3)189-197
Published online March 29, 2023, http://dx.doi.org/10.14283/jfa.2023.20
Abstract
BACKGROUND: The number of people aged 80 years and older (80+) will increase drastically in the upcoming decades. The preservation of cognitive functions will contribute to their quality of life and independence.
OBJECTIVES: To identify determinants of cognition and predictors of change in cognitive performance in the population 80+.
DESIGN: Cross-sectional and longitudinal population-based on the representative NRW80+ survey.
SETTING: Randomly drawn cases of people aged 80+ from the municipal registration offices, including people living in private homes and institutional settings.
PARTICIPANT: The participants in the cross-sectional sample (N=1503, 65.5%female) were 84.7 years old (95%CI[84.5,85.0]) and had 12.3 years of education (95%CI[12.1,12.4]). The participants in the longitudinal sample (N=840, 62.5%female) were 84.9 years old (95%CI[84.6,85.2]) and had 12.3 years of education (95%CI[12.0,12.5]).
MEASUREMENTS: The cognitive screening DemTect, age, sex, education, and social, physical, and cognitive lifestyle activities, as well as subjective general health status and depressive symptoms, were assessed at baseline and 24-month follow-up.
RESULTS: Younger age, more years of education, and more cognitive lifestyle activities were identified as the most consistent determinants of both better cognitive performance and preservation of cognitive performance for both global cognition as well as the DemTect subtests on memory and executive functions.
CONCLUSIONS: Our findings reveal that commonly investigated determinants of, and change in, cognitive performance are valid for the people 80+ and highlight the importance of cognitive lifestyle activities for cognitive health. The maintenance of cognitive functions is a key aspect of healthy aging in terms of preserving independence in people 80+.
Key words: Very old age, cognitive reserve, successful aging, prognostic research.
Introduction
The proportion of people aged 80 years and older (80+), sometimes called the oldest-old (1), is growing faster than any other segment of the older population. In 2000, there were 71 million people aged 80+ worldwide, which increased to 125 million in 2015. These numbers are projected to triple by 2050 (2). Whether longevity is perceived as a curse or a blessing largely depends on the extent to which this increasing life expectancy equals an extended period of good health (3). If decreases in physical and mental functions characterize these years, the burden for these individuals, their partners and caregivers, and society is immense (3, 4). However, experiences of good health can “add life to years,” as reported by the World Health Organisation (WHO, 2012) in their global brief on healthy aging.
In terms of preserving the independence of the people aged 80+ in daily life, one key aspect of healthy aging is the maintenance of cognitive functions. Cognitive decline progressively occurs with age in various cognitive domains, and it is important to note that decline in cognitive functions does not always equal pathological changes (5). The prevalence of clinically significant cognitive decline, such as mild cognitive impairment (MCI) or dementia (e.g., in the context of Alzheimer’s and Parkinson’s disease), is expected to increase rapidly (6). This increase constitutes a considerable threat to our health care system (7), especially when cognitive decline coincidences with other diseases in terms of multimorbidity and impaired general health status. Depressive symptoms represent another major factor and are strongly correlated with perceived subjective health, quality of life, and cognitive functioning in the people aged 80+ (8, 9).
Previous research shows that increasing age is one of the main risk factors for cognitive decline. Several different theoretical approaches are used to explain age-related cognitive decline (e.g., the disuse hypothesis, a decline in processing speed and the ability to inhibit task-irrelevant information) (5). The effect of age on cognition across the lifespan is not linear: especially in people aged 80+ an accelerated decline of cognitive functioning can be observed (10).
As in younger and young-old age (11), sex differences in cognition also exist in people aged 80+. This is despite a higher vulnerability for dementia in women compared to men (7), where women perform superior in general cognitive functioning, episodic memory tasks, and a variety of processing speed tasks (12, 13). However, the investigation of sex differences in cognition of people aged 80+ can be obscured by different methodological approaches, such as non-consistent sets of covariates (e.g., education). Previous analyses revealed an increased vulnerability for cognitive decline of women compared to men in people aged 80+ (14, 15). Results from longitudinal studies suggest that sex differences not only appear in cross-sectional studies but also influence the trajectories of cognitive decline. For example, a steeper decline in global cognitive functioning, processing speed, and visuo-cognition was observed in men when compared to women, and in contrast, no significantly steeper decline in any cognitive domain was observed for women compared to men (16).
The concept of cognitive reserve, which refers to “the adaptability of cognitive processes that helps to explain differential susceptibility of cognitive abilities or day-to-day function to brain aging, pathology, or insult“ (17), is frequently discussed in the context of healthy aging and cognition. Cognitive reserve can be operationalized by easy-to-administer socio-behavioral proxies such as education, occupational complexity, and engagement in cognitively stimulating lifestyle activities. As the meta-analysis by Opdebeeck, et al. (18) revealed, all these three socio-behavioral proxies of cognitive reserve were robustly associated with cognitive functioning in healthy older people, and each individual proxy contributed uniquely to cognitive reserve. Notably, findings from the Newcastle 85+ study indicated that a higher cognitive reserve was associated with better baseline global and domain-specific cognitive function, where cognitive reserve was operationalized by a composite score incorporating information on education, social class, marital status, engagement in mental activities, social participation, and physical activity (Lavrenic et al., 2018). The authors also found a reduced risk of prevalent dementia in people aged 80+. However, cognitive reserve did not influence trajectories of cognitive functioning across five years (19).
In contrast, Armstrong, et al. (20) did find relationships between greater initial leisure activity engagement (at the time of baseline assessment) and subsequent cognitive changes in their longitudinal sample of community-dwelling older adults. They observed a negative correlation of initial physical activity with subsequent memory decline (i.e., the higher the physical activity at baseline, the less memory decline was observed) during the study period. Furthermore, they found an inverse relationship (i.e., initial memory predicted later social leisure activity engagement) but not vice versa. Given the reciprocal interplay between leisure activity engagement and cognitive performance observed in their data, Armstrong, et al. (20) concluded that the relationship between the two constructs could possibly be explained by external common factors such as age-related brain changes. Regarding people aged 80+, other research has found no significant relationship between lifetime occupational complexity and cognition (21). In light of these heterogenous findings, which are partly due to inconsistent operationalizations, it seems worth examining the relationship between cognitive reserve and cognition in people aged 80+, especially in large representative samples.
Research on cognition in people aged 80+ is crucial given the expected increase in this population segment’s size in the upcoming decades and its anticipated societal impact. However, as reviewed in Giulioli and Amieva (22), there is a lack of research on cognition in this age group. In the present study, we aim to investigate (i) the determinants of cognition in people aged 80+ and (ii) predictors of change in cognitive performance over a two-year period. Our research is based on the large population-based representative survey used in the NRW80+ study titled the “Quality of life and subjective well-being of the very old in North Rhine-Westphalia” (23). We hypothesize that age, sex, education, social, physical, and cognitive lifestyle activities, subjective general health, and depressive symptoms are significant determinants of cognition (cross-sectional analyses) and predictors for change in cognitive performance over two years (longitudinal analyses) in people aged 80+.
Methods
Study design and setting
The present analyses utilize data from the 1st and 2nd wave of the NRW80+ study (23), a population-based, representative survey conducted in North Rhine-Westphalia, Germany’s most populous state, from August 2017 to February 2018 (1st wave) and from June 2019 to February 2020 (2nd wave). The study was approved by the ethics committee of the Faculty of Medicine of the University of Cologne (vote-no. 17–169). Written informed consent was obtained from all participants prior to study participation. A detailed description of design decisions and the conceptual framework has been published by Hansen, et al. (24). Outcomes of the NRW80+ study were mainly based on the Challenges and Potentials Model of Quality of Life in Very Old Age (CHAPO) framework (23). Reporting of the present analyses follows the STROBE guidelines (25).
Participants
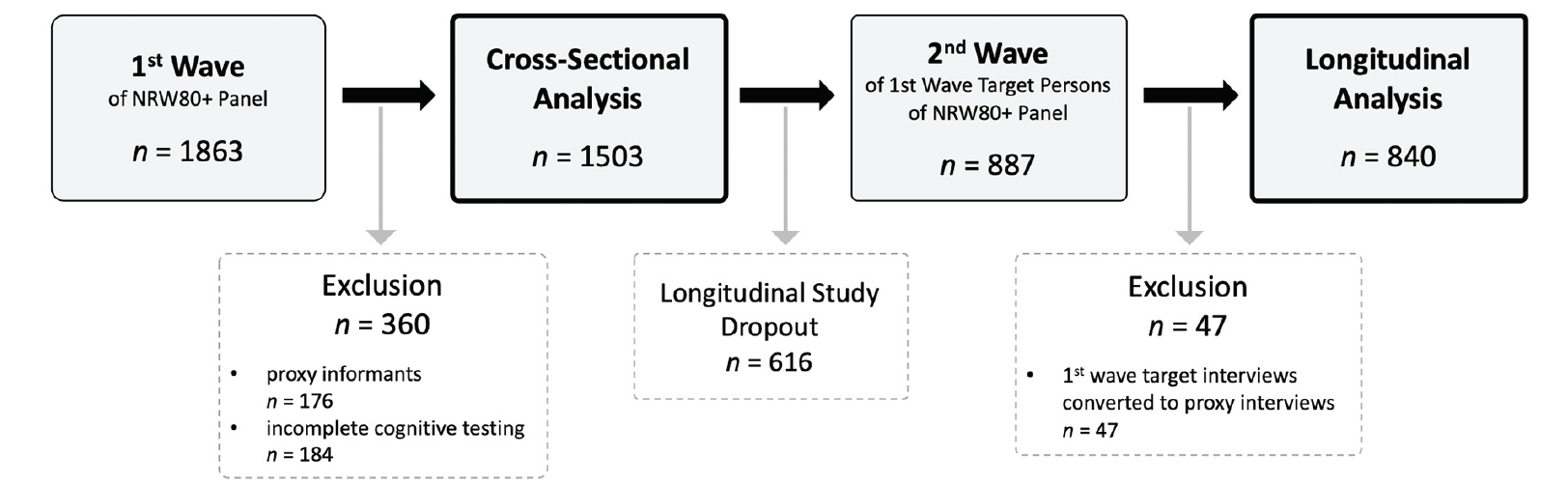
The initial NRW80+ study sample included N = 1,863 randomly drawn cases of people aged 80+ from the municipal registration offices living in both private homes and institutional settings. The computer-assisted personal interviews were conducted by experienced and trained interviewers of Kantar (previously TNS Infratest, Munich, Germany). For the present analyses, the sample was selected as outlined in Figure 1. The cross-sectional analyses on the determinants of cognition were performed on N = 1,503 individuals from the 1st wave, while the cognitive screening was conducted with N = 840 targeted persons in the 2nd wave, constituting the sample for the longitudinal analyses on predictors for change in cognitive performance across two years.
The NRW80+ study sample consists of N = 1863 randomly drawn cases of people aged 80+. In case people were not able to participate in the interview in person due to health reasons, a proxy interview was conducted (N = 176). In the case of a proxy interview, however, some assessments including the cognitive screening of the target persons were not carried out. The cross-sectional analyses on determinants of cognition were performed on N = 1503 persons of the 1st wave of the panel. Participants of the 1st wave who indicated that they want to participate in subsequent waves were contacted two years later for participation in the 2nd wave. For N = 887 target persons from the 1st wave, longitudinal data was available. N = 47 target person interviews from the 1st wave converted to proxy interviews in the 2nd wave. Following, cognitive screening was conducted with N = 840 target persons in the 2nd wave, constituting the sample for the longitudinal analyses on predictors for change in cognitive performance across two years.
Outcomes and predictors
For the cross-sectional and longitudinal analyses, the primary outcome was the total score of the DemTect cognitive screening (26). The DemTect subscores constitute the secondary outcomes. The cross-sectional analyses assessed several variables as determinants of cognition, and the longitudinal analyses assessed variables as predictors for change in cognitive performance.
Cognitive Assessment
The DemTect Version A was administered to target persons as a cognitive screening instrument (26, 27). Subtests of the DemTect include (i) Word List Learning, (ii) Number Transcoding, (iii) Semantic Verbal Fluency (“Supermarket”), (iv) Digit Span Backwards, and (v) Word List Delayed Recall. Maximum raw scores for each of the subtests are reported in Table 1. The transformed total score of the DemTect test (maximum of 18 points), which is a sum of the transformed raw scores of each subtest, is corrected for age and education. The range for age-adequate cognitive functioning is 13–18 points, for MCI 9–12 points, and ≤8 points indicate a high likelihood of dementia (26). For the analyses on DemTect subtests in the present manuscript, raw scores of the five subtests were used.
Determinants and Predictors
Age in years, as reported by municipal offices during sampling for the 1st wave, was included as a determinant and predictor in the cross-sectional and longitudinal analyses, respectively. Sex was coded as a binary variable (0 = male, 1 = female). Education was operationalized as total years of primary and secondary school education, university education, and professional training. Data on the highest school-leaving qualification and the highest completed professional training were recoded to the standard number of years for completing the respective degree, according to Hoffmeyer-Zlotnik (28).
Social, physical, and cognitive lifestyle activities were assessed by asking participants whether they had engaged in 17 different activities over the past 12 months (0 = no, 1 = yes) and the corresponding frequency of performance (1 = daily, 2 = weekly, 3 = monthly, 4 = several times a year, 5 = once a year) if applicable. Lifestyle activities were grouped into three domains (i) social, (ii) physical, and (iii) cognitive lifestyle activities. For details on the selection and allocation of lifestyle activities evaluated in the NRW80+ study, please refer to the Supplementary Material. For each domain, a composite score was computed as the number of lifestyle activities in the respective domain per day, with higher scores indicating more activities. In the cross-sectional analyses, the scores for social, physical, and cognitive lifestyle activities during the 1st wave were examined as determinants for cognitive performance. In the longitudinal analyses, the scores for social, physical, and cognitive lifestyle activities during the 2nd wave were evaluated as predictors for change in cognitive performance over two years.
Subjective general health was assessed on a 4-point Likert scale (1 = very bad, 2 = rather bad, 3 = rather good, 4 = very good), and higher scores indicated better subjective general health.
Depressive symptoms were assessed with the 4-item short-form of the Depression in old Age Scale (DIA-S4) (29, 30). The four questions evaluate the presence of four key symptoms of depression, (i) depressed mood, (ii) low energy, (iii) loss of pleasure, and (iv) rumination. The maximum score was 4, with higher scores indicating more depressive symptoms. Scores of 2 or greater represent clinically meaningful depressive symptoms (29, 30).
Bias
Survey weights were utilized to address potential selection bias in the cross-sectional sample. These weights accounted for various factors such as age group, sex, household size, regional area, type and size of the community, housing form (private or institutionalized), and marital status. To address panel bias, longitudinal weights were used. These weights corrected for potential biases regarding cognitive status, activities of daily living, household size, overall health rating, housing form (private or institutionalized), age group, sex, social status, and urbanization.
Statistical analyses
We used SPSS 28 for data handling and Stata 17.0 for data analyses. The sample description reports means and 95% confidence intervals (CI) for continuous variables, and percentages and 95%CI for continuous variables. Multiple linear regression models were estimated in the cross-sectional and longitudinal analyses. The dependent variables were the total DemTect scores and the raw scores of the subtests of the DemTect at the 1st wave for the cross-sectional analyses. For the longitudinal analyses, we used difference scores between the two waves [Δ(2nd wave–1st wave)] of the total DemTect score and the raw scores of the DemTect subtests as dependent variables. Age, sex, education, physical, social, and cognitive lifestyle activities, subjective general health status, and depression were entered as determinants and predictors.
For the longitudinal analyses, we also included the corresponding baseline score of the 1st wave in the regression model. Tests for multicollinearity did not exceed critical values (Variance Inflation Factor <5). To measure the change in cognitive performance between the two waves in the longitudinal sample, we used dependent sample t-tests. Cohen’s d was reported as the effect size, and we adopted Brydges (31) classification for effect sizes in gerontological settings, indicating small (≥ 0.15), medium (≥ 0.4) or large (≥ 0.7) effects.
Overall, 3.2% of the data were missing in the cross-sectional data and 6.4% in the longitudinal data. For the cognitive variables, missing data approximated 5.6% (cross-sectional) and 9.8% (longitudinal). Missing values were assumed to be missing at random. To impute the missing values, a fully conditional specification approach with twenty imputed datasets was used. To ensure a good prediction of missing values, we excluded cases with less than three completed subtests of the DemTect. Pooled R2 were calculated with Fisher’s z-transformation and retransformation. The alpha level was set at .05. Analyses of the DemTect test total score indicating global cognitive performance were regarded as confirmatory, and related p-values were corrected with the Bonferroni–Holm procedure to control the family-wise error rate of 5%. However, for the subtests of the DemTect representing cognitive performance in separate cognitive domains, we adopted an exploratory approach with unadjusted p-values.
Results
Sample characteristics
Characteristics of participants in both the cross-sectional (N = 1,503) and the longitudinal (N = 840) sample are displayed in Table 1. The participants in the cross-sectional sample were on average 84.7 years old (95%CI[84.5,85.0]), averaged 12.3 years of education (95%CI[12.1,12.4]), and 63.1% (95%CI[60.6%,65.5%]) were female. In the cross-sectional sample, 71.8% (95%CI[69.6,74.1]) of participants scored age-adequately in the DemTect, 17.4% (95%CI[15.5,19.4]) could be classified as MCI, and 10.8% (95%CI[9.3,12.5]) fell in the range for dementia.
Table 1. Sample Description of the NRW80+ Study Sample for the Cross-Sectional (1st Wave) and the Longitudinal Analyses (1st and 2nd Wave)
Notes. Data are mean (95% confidence interval) unless indicated otherwise. *The range for age-adequate cognitive functioning in the DemTect is 13–18 points, for MCI 9–12 points, and ≤ 8 points indicate a high likelihood for dementia (26, 27).
The longitudinal sample consisted of participants with a mean age of 84.9 years (95%CI[84.6,85.2]), 12.3 years of education on average (95%CI[12.0,12.5]), and 62.5% (95%CI[58.7%,66.4%]) were female. 71.5% (95%CI[68.5,74.6]) scored age-adequately in the DemTect test, 17.8% (95%CI[15.3,20.6]) could be classified as MCI, and 10.7% (95%CI[8.7,13.0]) fell in the range for dementia.
Cross-sectional analyses: Determinants of cognitive performance
In the multiple linear regression model, better overall cognitive performance (measured using the DemTect total score) was significantly associated with younger age, higher education, and increased levels of cognitive lifestyle activities. No significant associations were found between cognitive performance and sex, social and physical lifestyle activities, subjective health, and depression. The multiple linear regression model is presented in Table 2.
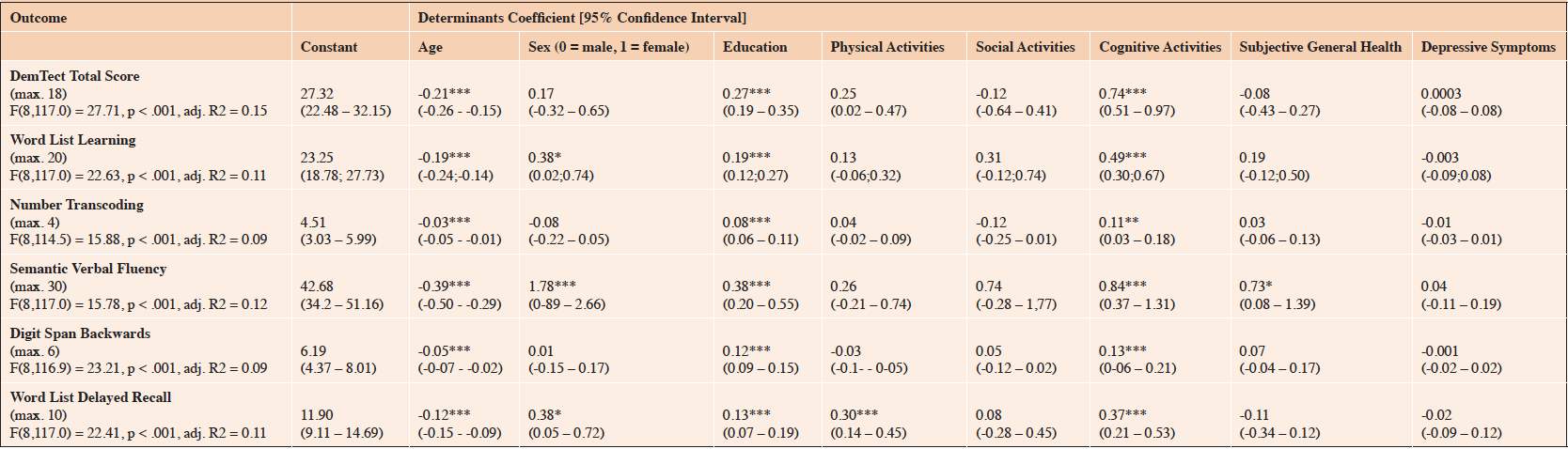
Table 2. Results of the Cross-Sectional Multiple Linear Regression Models on Determinants of Cognitive Functioning in the Oldest-Old during the 1st Wave of the NRW80+ Study (n = 1503)
Notes. Asterisks indicate Bonferroni-Holm-corrected levels of significance. *** p ≤ .001; ** p ≤ .010; * p ≤ .050
Younger age and higher education predicted better performance in each of the five subtests of the DemTect. Furthermore, sex was found to be a significant determinant for cognitive performance in three DemTect subtests. Beyond the other determinants, female sex was significantly associated with better scores in the DemTect subtests of Word List Learning, Semantic Verbal Fluency, and Word List Delayed Recall. Higher levels of cognitive lifestyle activities were associated with better scores in all DemTect subtests, while higher levels of physical lifestyle activities were only associated with better performance in the subtest of Word List Delayed Recall. Social lifestyle activities and depression did not show significant associations with cognitive performance in any subtest. Finally, better subjective general health was found to be significantly associated with better performance in the Semantic Verbal Fluency subtest.
Longitudinal analyses: Predictors of change in cognitive performance
Over the two-year period between the 1st and the 2nd wave, the performance in the DemTect test total score, (t(82.73) = -3.89, p < .001, Cohen’s d = 0.177), the Digit Span Backwards subtest, (t(107.13) = -2.60, p = .011, Cohen’s d = 0.121), and the Word List Delayed Recall subtest, (t(100.38) = -3.22, p = .002, Cohen’s d = 0.156) significantly decreased. Performance in the subtests of Word List Learning, (t(100.19) = -1.96, p=.053, Cohen’s d = 0.092) and Semantic Verbal Fluency, (t(111.37) = -1.96, p = .052, Cohen’s d = 0.111 did not decrease on the 5% level, but on a 10% alpha level. Number Transcoding did not decrease significantly over the two-year period, (t(46.3) = -0.53, p = .599, Cohen’s d = 0.032). Figure 2 visualizes the cognitive performance in the DemTect for the longitudinal sample in the 1st and 2nd waves.
The change in the DemTect total score between the 1st and the 2nd wave was significantly associated with the DemTect total baseline score, age, and the frequency of cognitive lifestyle activities. Worse performance at the 1st wave and younger age were significantly associated with less decline in the DemTect total score. Higher levels of cognitive lifestyle activities were significantly associated with less cognitive decline. The model is presented in Table 3.
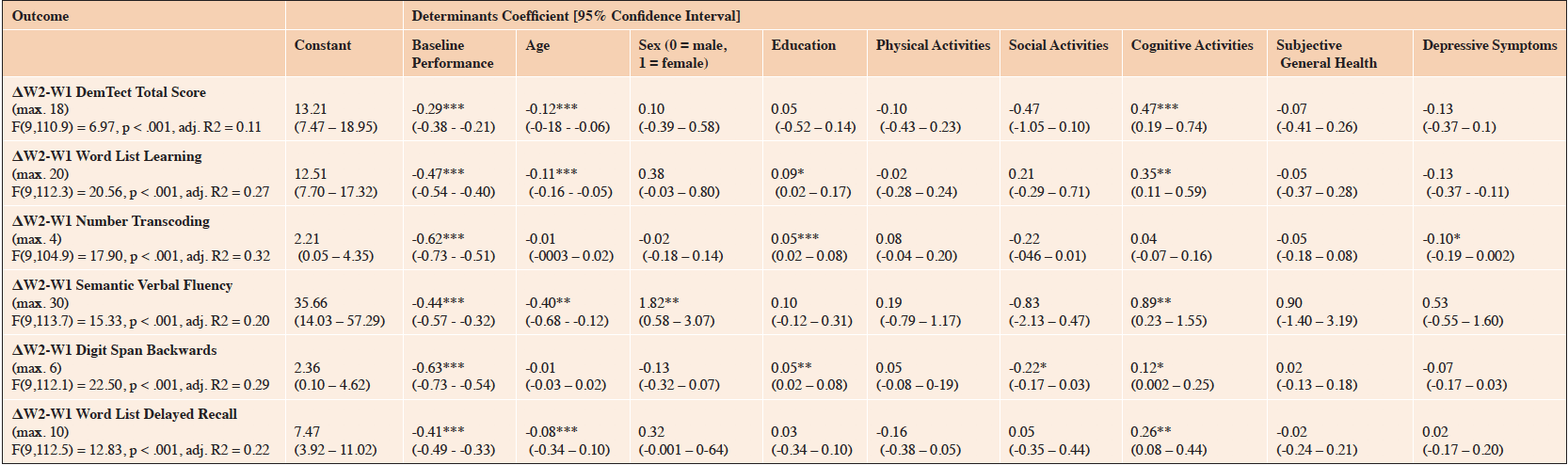
Table 3. Results of the Longitudinal Multiple Linear Regression Models on Predictors of Change in Cognitive Functioning in the Oldest-Old between the 1st Wave and the 2nd Wave of the NRW80+ Study (n = 840)
Notes. ΔW2-W1, Difference score between the 1st wave and the 2nd wave in the NRW80+ study. Asterisks indicate Bonferroni-Holm-corrected levels of significance. *** p ≤ .001; ** p ≤ .010; * p ≤ .050
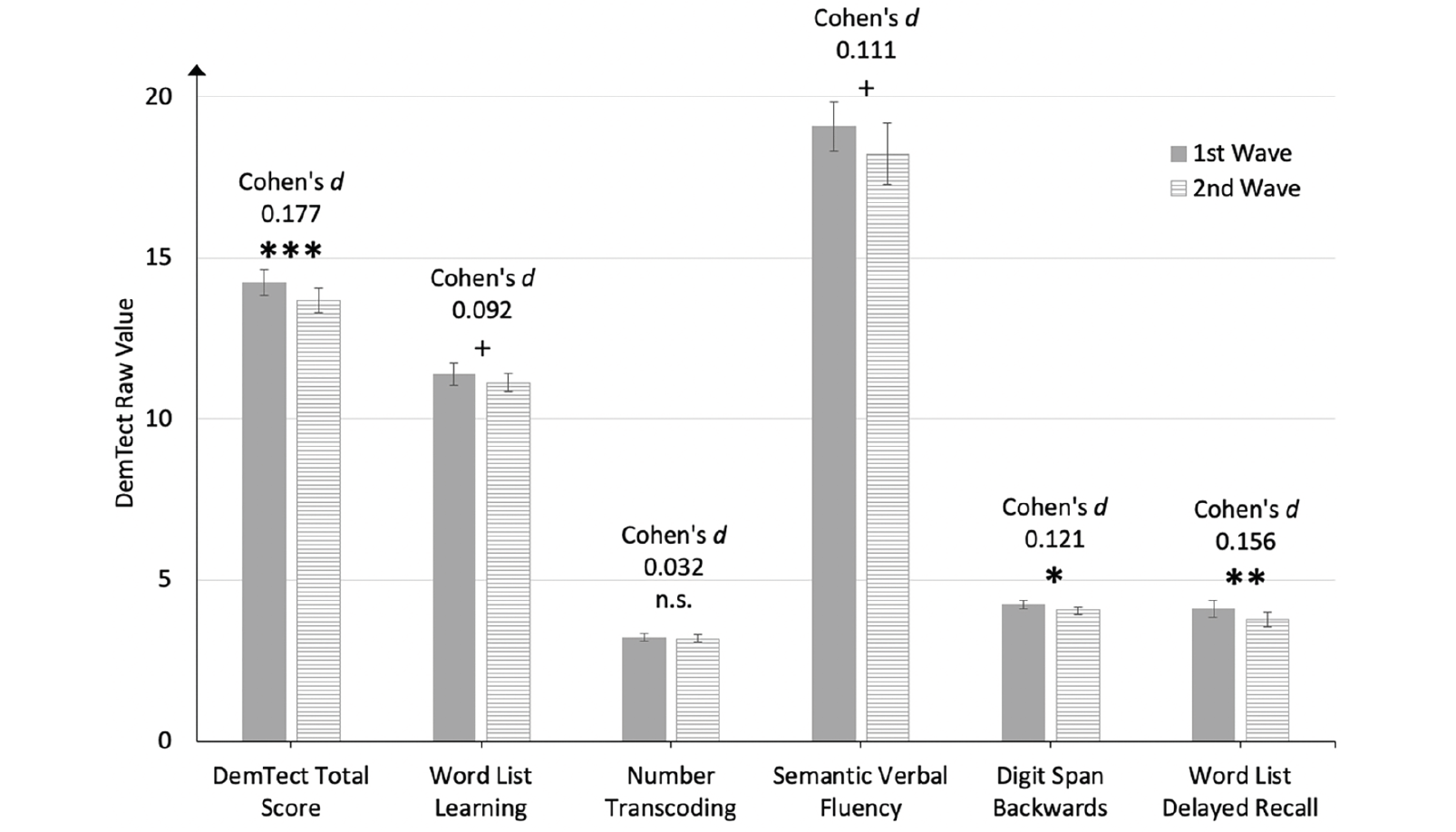
Figure 2. Cognitive Performance in the DemTect of the Longitudinal Sample (n = 840) in the 1st wave and the 2nd wave in the NRW80+ Study
Error bars indicate 95% confidence intervals; Asterisks indicate Bonferroni-Holm-corrected levels of significance. *** p < .001; **p < .010; * p < .050; + < .010
For all the DemTect subtests, baseline performance at the 1st wave significantly predicted the change in cognitive performance, indicating that worse performance at baseline was associated with less decline. Higher levels of cognitive lifestyle activities predicted less decline in the DemTect subtests of Word List Learning, Semantic Verbal Fluency, Digit Span Backwards, and Word List Delayed Recall. Fewer social lifestyle activities were associated with more decline in working memory, and physical lifestyle activities were not significantly associated with any change in cognitive performance.
Younger age predicted the lesser worsening in the DemTect subtests of Word List Learning, Semantic Verbal Fluency, Digit Span Backwards, and Delayed Word List Recall. Sex was only identified as a significant predictor for change in the subtest of Semantic Verbal Fluency, with female sex being associated with better cognitive outcomes when compared to males. Higher education was associated with less cognitive decline in the DemTect subtests of Word List Learning, Number Transcoding, and Digit Span Backwards. Fewer depressive symptoms were associated with more decline in the DemTect subtest of Number Transcoding. For subjective general health, no significant independent relationship to cognitive change was observed between the 1st and 2nd waves in any of the DemTect subtests. The complete models are presented in Table 3.
Discussion
The present analyses were conducted using a large cohort of individuals aged 80+ from the NRW80+ study (23). The analyses aimed to evaluate determinants of cognition in this population by analyzing a cross-sectional sample and predictors of change in cognitive performance by analyzing a longitudinal sample over a two-year period. The main finding from the cross-sectional analyses of cognition in this age group is that (i) younger age, more years of education, and greater engagement in cognitive lifestyle activities were consistently and independently associated with better cognitive performance in global cognition and all cognitive domains evaluated in the DemTect (26) (i.e., including short- and long-term memory, working memory, and executive functions). In addition, we found that (ii) female sex correlated with better performance in short-term memory and executive functions.
Our longitudinal analyses of cognitive performance over the two-year period in people aged 80+ revealed the following key findings: (i) Cognitive performance significantly declined across the two-year period, (ii) this decline was more pronounced in those with higher initial cognitive performance, (iii) younger age and greater engagement in cognitive lifestyle activities were associated with less cognitive decline across multiple cognitive domains, including global cognition, short- and long-term memory, and executive functions, (iv) more years of education and greater engagement in cognitive lifestyle activities were associated with less decline in working memory, and finally, (v) more years of education were associated with less cognitive decline in short-term memory and executive functions. Furthermore, female sex predicted less decline in executive functions over the two-year period.
The most consistent determinants for present cognitive performance and predictors for change of cognitive performance in global cognition and separate cognitive domains across the two years were age, education, and cognitive lifestyle activities. Even within people 80+, age emerged as a significant independent determinant of both present cognitive performance and the rate of decline within two years of time. As reviewed by Paraskevoudi, et al. (32), extensive changes in the central nervous and musculoskeletal systems that lead to broad changes in motor, sensory, cognitive, and temporal processing, make age one of the major risk factors for dementia in people aged 80+. However, the shape of cognitive trajectories in the oldest old is discussed controversially, as both linear (33) and exponential trajectories (34, 35), potentially resulting in a terminal decline [10], are reported. Since there were only two points of time in the longitudinal analyses, we were unable to contribute to this discussion, as conclusions derived from mixing cross-sectional and longitudinal findings would not have been reliable. Future studies investigating cognition in people aged 80+ should incorporate multiple follow-up assessments. This would allow the fitting of within-subject trajectories of cognitive performance across time in large representative samples.
Female sex was a positive predictor for cognitive performance in the DemTect subtests of Word List Learning and Recall and Semantic Verbal Fluency. These subtests rely on memory functions (episodic and semantic memory), with the verbal fluency test requiring executive functions. This indicated that women performed superior to men in these domains, which is well established in the general ageing process (11), and even at a very advanced age (12, 13). Furthermore, female sex predicted less decline in the DemTect Semantic Verbal Fluency Subtest, indicating a potentially protective influence, even in the long term. It appears somehow counterintuitive that women outperform men in certain cognitive tasks despite their increased risk for dementia (36), which is indeed not fully understood to date. The longer life expectancy of women compared to men certainly contributes to a higher lifetime prevalence of dementia in women compared to men, however, even age-adjusted prevalence rates are higher in women compared to men (36). In general, the higher dementia risk is discussed to be related to women having fewer modifiable risk factors over the lifespan (37). However, there is also a complex relationship between protective aspects for dementia potentially resulting in “memory resilience” (38), that is preserved memory performance despite undeniable (e.g., genetic) risk factors.
The identification of positive associations between (i) the number of years of education and cognitive lifestyle activities and (ii) the cognitive performance and the preservation of cognitive abilities across time point to the concept of cognitive reserve (17). Consistent with findings from the Newcastle 85+ study (19), we found a consistent association between a higher cognitive reserve and cognitive performance. However, Lavrencic, et al. (19) did not identify a relationship between cognitive reserve and trajectories of cognitive functioning over five years. Our analyses revealed an independent and protective influence of more educational years and cognitively stimulating lifestyle activities (e.g., playing board games, continuing education, and brain games / cognitive training) on cognitive performance in people aged 80+ over the two years. The operationalization of cognitive reserve varies widely between studies, which may be one explanation for these heterogeneous findings (18).
Within our analyses, cognitive lifestyle activities were the most consistent determinant of the change in cognitive performance regarding global cognition as measured with the DemTect total score and performance in the DemTect subtests representing the cognitive domains of memory and executive functions, including working memory. We evaluated the scores for social, physical, and cognitive lifestyle activities during the assessments of the 2nd as predictors for change in cognitive performance over two years, since they were considered more valid proxy variables for the actual level of lifestyle activities performed during the time of study compared to the assessed level during the 1st wave of assessments. Assessing cognitive lifestyle activities in this way ensured an understanding of cognitive reserve as a dynamic construct that is continuously shaped by lifetime exposure to contributing factors (17). It is argued that each contributing socio-behavioral proxy (e.g., education and different lifestyle activities) may uniquely contribute to cognitive reserve, which was supported by our data.
Armstrong, et al. (20) found an inverse relationship between cognitive performance and lifestyle activities, which was not investigated within our analyses. In this context, the limits of multiple linear regression analyses should be discussed, as they are unable to directly explore causal relationships (39). The widely-applied and well-accepted argument of temporal precedence does not always correspond to true causal mechanisms (39). Nevertheless, as we simultaneously investigated the influence of the total years of education and cognitive lifestyle activities in one regression model, we were able to reduce the risk of unmeasured confounding and inverse causation in the context of education, cognitively stimulating lifestyle activities, and actual cognitive performance (40). Furthermore, while the approach of Armstrong, et al. (20) measures cognition with one global factor, a strength of our approach is to keep observable and relevant clinical constructs as outcome measures.
One main limitation of the present analyses is that they rely on a single cognitive screening instrument. The DemTect (26) was developed to efficiently screen for the early cognitive symptoms of dementia and MCI. However, the DemTect primarily focuses on mnestic and executive functions and does not cover visuo-cognition, attention, or pure language functions. The addition of these domains would complement the range of cognitive domains typically assessed in clinical contexts (e.g., in the diagnostic workflow or when investigating cognitive profiles in research settings). Nevertheless, the use of a brief, easy-to-administer cognitive screening tool within the NRW80+ study allowed for the complementation of the interdisciplinary assessment of a broad range of variables, including objective social micro and macro conditions, subjective well-being, self-reported and tested health conditions, and, where possible, objective biological markers (23). Another limitation refers to the relatively short period between the 1st and the 2nd wave of the NRW80+ study (24 months), and the fact that only two assessments were conducted, which limits the conclusions that can be drawn from the trajectories of cognitive performance and health in people aged 80+. Future studies with more and longer follow-ups are necessary to address this limitation.
One major strength of the present analyses is that our analyses were based on the large representative sample of the NRW80+ study (23), providing representative insights into cognition in people 80+ in Germany’s most populous state. The state-of-the-art methodology regarding the implementation of the present survey project incorporates substantial efforts to reduce selectivity and increase representativity in the sample. Initial cross-sectional and longitudinal weights addressed panel bias from various socio-economic and health-related variables. In both samples, about 11% of individuals were classified as having dementia, indicating that the inclusion of this vulnerable and hard-to-access group was successful. The similar percentages of people with dementia in the cross-sectional and the longitudinal sample suggests that both samples were representative of people with dementia and, importantly, not affected by longitudinal dropout. However, the inclusion criteria for the present analyses contributed to a decrease regarding this representativity as the DemTect was not conducted in proxy interview scenarios, therefore, excluding cases for which proxy interviews were conducted. This contributed to increased dropout from the 1st to the 2nd wave if target person interviews from the 1st wave converted to proxy interviews in the 2nd wave, adding to the longitudinal dropout. Consequently, longitudinal data for the present analyses was only available for 55.89% of 1st wave target persons (40.98% longitudinal dropout, 3.13% additional proxy converter dropout). However, models underlying the calculation of longitudinal weights included cognitive status as one predictor, which reduces bias resulting from the exclusion of proxy interviews and from panel selection (41). As global cognitive functioning is a major determinant of older people’s independence (42), the present analyses may underestimate the progression of cognitive decline in people 80+.
With the present analyses, we substantially contribute to the field of research on cognition in people aged 80+, which might amend our understanding of cognitive health across the lifespan. Our findings reveal that commonly investigated determinants of cognitive performance and change in cognitive performance are also valid for the sample of people aged 80+ and highlight the importance of cognitive lifestyle activities for cognitive health across the lifetime. Facing the societal challenge of age-related demographic change and the corresponding goal to “add life to years” (43), these findings should motivate younger generations to promote healthy, active aging not only in the younger older adults, but also in people aged 80+.
Acknowledgements: The project NRW80+ belongs to the Key Research Area “Aging and Demographic Change” (Speaker: Susanne Zank) of the Cologne Center for Ethics, Rights, Economics, and Social Sciences of Health (ceres) which is directed by Michael Wagner at the University of Cologne. Members of the project board are Michael Wagner, Christiane Woopen and Susanne Zank. The data, code book and materials of the NRW80+ Study are openly available under https://doi.org/10.4232/1.13978, Zank et al., 2022. The analysis code for the present analyses can be requested from the corresponding author upon reasonable request.
Funding: The project NRW80+ is funded by the Ministry of Innovation, Science and Research, North Rhine-Westphalia. Funding note: Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest: TB, AC, SZ: none. AO has received a grant from the Koeln Fortune Program (grant-no. 329/2021) and a grant of the Novartis-Stiftung für therapeutische Forschung 2021, both outside the submitted work. AKF has received honoraria from Springer Medizin Verlag GmbH, Heidelberg, Germany; Springer-Verlag GmbH, Berlin; ProLog Wissen GmbH, Cologne, Germany; Bundesverband Klinische Linguistik e.V., Coburg, Germany; Hochschule Fresenius, Düsseldorf, Germany; as well as Seminar- und Fortbildungszentrum Rheine, Germany; and has received grants from the German Parkinson Society; German Alzheimer’s Society; Federal Joint Committee (G-BA); and STADAPHARM GmbH. EK: EK has received honoraria from ProLog Wissen GmbH, Cologne, Germany; Kyowa Kirin Services LTD, London, United Kingdom; AbbVie Inc., as well as from the Movement Disorders Society; and has received grants from German Ministry of Education and Research (BMBF); German Parkinson Society; German Alzheimer’s Society; Federal Joint Committee (G-BA); and STADAPHARM GmbH.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Degnen C (2007) Minding the gap: The construction of old age and oldness amongst peers. J. Aging Stud. 21, 69-80, https://doi.org/10.1016/j.jaging.2006.02.001.
2. United Nations (2015) World population ageing. UN DESA,
3. Beard JR, Officer A, De Carvalho IA, Sadana R, Pot AM, Michel J-P, Lloyd-Sherlock P, Epping-Jordan JE, Peeters GG, Mahanani WR (2016) The World report on ageing and health: a policy framework for healthy ageing. The lancet 387, 2145-2154,
4. Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL (2019) Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. The Lancet Public Health 4, e159-e167,
5. Salthouse TA (2016) Theoretical perspectives on cognitive aging, Psychology Press.
6. Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, Abdoli A, Abualhasan A, Abu-Gharbieh E, Akram TT (2022) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. The Lancet Public Health 7, e105-e125,
7. GBD 2016 Dementia Collaborators (2019) Global, regional, and national burden of Alzheimer’s Disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 88-106, 10.1016/S1474-4422(18)30403-4.
8. Bergdahl E, Gustavsson JM, Kallin K, von Heideken Wågert P, Lundman B, Bucht G, Gustafson Y (2005) Depression among the oldest old: the Umeå 85+ study. Int. Psychogeriatr. 17, 557-575, https://doi.org/10.1017/S1041610205002267.
9. Stek M, Gussekloo J, Beekman A, van Tilburg W, Westendorp R (2004) Prevalence, correlates and recognition of depression in the oldest old: the Leiden 85-plus study. J. Affect. Disord. 78, 193-200, https://doi.org/10.1016/S0165-0327(02)00310-5.
10. Johansson B, Hofer SM, Allaire JC, Maldonado-Molina MM, Piccinin AM, Berg S, Pedersen NL, McClearn GE (2004) Change in cognitive capabilities in the oldest old: the effects of proximity to death in genetically related individuals over a 6-year period. Psychol Aging 19, 145, https://doi.org/10.1037/0882-7974.19.1.145.
11. Munro CA, Winicki JM, Schretlen DJ, Gower EW, Turano KA, Muñoz B, Keay L, Bandeen-Roche K, West SK (2012) Sex differences in cognition in healthy elderly individuals. Aging, Neuropsychology, and Cognition 19, 759-768,
12. Gerstorf D, Herlitz A, Smith J (2006) Stability of sex differences in cognition in advanced old age: the role of education and attrition. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 61, P245-P249,
13. Van Exel E, Gussekloo J, De Craen A, Bootsma-Van Der Wiel A, Houx P, Knook D, Westendorp R (2001) Cognitive function in the oldest old: women perform better than men. Journal of Neurology, Neurosurgery & Psychiatry 71, 29-32,
14. Miyawaki CE, Liu M (2019) Gender differences in cognitive impairment among the old and the oldest-old in China. Geriatr Gerontol Int . 19, 586-592, https://doi.org/10.1111/ggi.13666.
15. Xie J, Matthews FE, Jagger C, Bond J, Brayne C (2008) The oldest old in England and Wales: a descriptive analysis based on the MRC Cognitive Function and Ageing Study. Age Ageing 37, 396-402, https://doi.org10.1093/ageing/afn061.
16. McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM (2016) Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging 31, 166, https://doi.org/10.1037/pag0000070.
17. Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS (2020) Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia 16, 1305-1311, 10.1016/j.jalz.2018.07.219.
18. Opdebeeck C, Martyr A, Clare L (2016) Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Aging Neuropsychol Cogn 23, 40-60, https://doi.org/10.1080/13825585.2015.1041450.
19. Lavrencic LM, Richardson C, Harrison SL, Muniz-Terrera G, Keage HA, Brittain K, Kirkwood TB, Jagger C, Robinson L, Stephan BC (2018) Is there a link between cognitive reserve and cognitive function in the oldest-old? J. Gerontol. A Biol. Sci. 73, 499-505, https://doi.org/10.1093/gerona/glx140.
20. Armstrong NM, Tom SE, Harrati A, Casaletto K, Pa J, Arce Rentería M, Gu Y, Rajan KB, Schupf N, Fieo R (2022) Longitudinal Relationship of Leisure Activity Engagement With Cognitive Performance Among Non-Demented, Community-Dwelling Older Adults. Gerontologist 62, 352-363, https://doi.org/10.1093/geront/gnab046.
21. Hakiki B, Pancani S, Portaccio E, Molino-Lova R, Sofi F, Macchi C, Cecchi F (2021) Impact of occupational complexity on cognitive decline in the oldest-old. Aging Ment Health 25, 1630-1635, https://doi.org/10.1080/13607863.2020.1746739.
22. Giulioli C, Amieva H (2016) Epidemiology of cognitive aging in the oldest old. Revista de investigación clínica 68, 33-39, https://pubmed.ncbi.nlm.nih.gov/27028175/.
23. Wagner M, Rietz C, Kaspar R, Janhsen A, Geithner L, Neise M, Kinne-Wall C, Woopen C, Zank S (2018) Quality of life of the very old. Z Gerontol Geriatr 51, 193-199, https://doi.org/10.1007/s00391-017-1217-3.
24. Hansen S, Kaspar R, Wagner M, Woopen C, Zank S (2021) The NRW80+ study: conceptual background and study groups. Z Gerontol Geriatr 54, 76-84, https://doi.org/10.1007/s00391-021-01970-z.
25. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453-1457, https://doi.org/10.1136/bmj.39335.541782.AD.
26. Kalbe E, Kessler J, Calabrese P, Smith R, Passmore A, Brand Ma, Bullock R (2004) DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. International journal of geriatric psychiatry 19, 136-143,
27. Kessler J, Fengler S, Kaesberg S, Müller K, Calabrese P, Ellwein T, Kalbe E (2014) DemTect 40–und DemTect 80+: Neue Auswertungsroutinen für diese Altersgruppen. Fortschritte der Neurologie· Psychiatrie 82, 640-645,
28. Hoffmeyer-Zlotnik JH (1997) in Kongress der Deutschen Gesellschaft für Soziologie» Differenz und Integration Campus Verl., pp. 908-925.
29. Heidenblut S, Zank S (2010) Development of a new screening instrument for geriatric depression. The depression in old age scale (DIA-S). Z Gerontol Geriatr 43, 170-176, https://doi.org/10.1007/s00391-009-0067-z.
30. Heidenblut S, Zank S (2020) Screening for depression in old age with very short instruments: the DIA-S4 compared to the GDS5 and GDS4. Gerontol. Geriatr. Med. 6, 2333721420981328, https://doi.org/10.1177/2333721420981328.
31. Brydges CR (2019) Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov Aging 3, igz036, https://doi.org/10.1093/geroni/igz036.
32. Paraskevoudi N, Balcı F, Vatakis A (2018) “Walking” through the sensory, cognitive, and temporal degradations of healthy aging. Annals of the New York Academy of Sciences 1426, 72-92, 10.1111/nyas.13734.
33. Lucca U, Tettamanti M, Logroscino G, Tiraboschi P, Landi C, Sacco L, Garrì M, Ammesso S, Bertinotti C, Biotti A (2015) Prevalence of dementia in the oldest old: the Monzino 80-plus population based study. Alzheimers. Dement. 11, 258-270. e253, https://doi.org/10.1016/j.jalz.2014.05.1750.
34. Corrada MM, Berlau DJ, Kawas CH (2012) A population-based clinicopathological study in the oldest-old: the 90+ study. Curr. Alzheimer Res. 9, 709-717, https://doi.org/10.2174/156720512801322537.
35. Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH (2010) Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann. Neurol. 67, 114-121, https://doi.org/10.1002/ana.21915.
36. Nichols E, Szoeke CE, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, Aichour MTE, Akinyemi RO, Alahdab F, Asgedom SW (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 88-106,
37. Anstey KJ, Peters R, Mortby ME, Kiely KM, Eramudugolla R, Cherbuin N, Huque MH, Dixon RA (2021) Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20–76 years. Scientific Reports 11, 7710,
38. McDermott KL, McFall GP, Andrews SJ, Anstey KJ, Dixon RA (2017) Memory resilience to Alzheimer’s genetic risk: Sex effects in predictor profiles. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 72, 937-946,
39. Wiedermann W, von Eye A (2015) Direction of effects in multiple linear regression models. Multivar. Behav. Res. 50, 23-40, https://doi.org/10.1080/00273171.2014.958429.
40. Sajeev G, Weuve J, Jackson JW, VanderWeele TJ, Bennett DA, Grodstein F, Blacker D (2016) Late-life cognitive activity and dementia: a systematic review and bias analysis. Epidemiology 27, 732, https://doi.org/10.1097/EDE.0000000000000513.
41. Brix J, Steinacker G, Schneekloth U (2021) NRW80+ Lebensqualität und Wohlbefinden älterer Menschen in Nordrhein-Westfalen – Methodenbericht, Kantar Public, München, Germany.
42. Hajek A, Luppa M, Brettschneider C, van der Leeden C, van den Bussche H, Oey A, Wiese B, Weyerer S, Werle J, Fuchs A (2021) Correlates of institutionalization among the oldest old—Evidence from the multicenter AgeCoDe-AgeQualiDe study. Int. J. Geriatr. Psychiatry 36, 1095-1102, https://doi.org/10.1002/gps.5548.
43. World Health Organization (2012) Good health adds life to years: Global brief for World Health Day 2012. WHO, https://apps.who.int/iris/handle/10665/70853.
The Author(s) 2023