A.B. Maier1,2,3, S.T.H. Chew4, J. Goh2,3,5, F.H.X. Koh6, N.C. Tan7
1. Faculty of Behavioural and Movement Sciences, Department of Human Movement Sciences, @AgeAmsterdam, Vrije Universiteit, Amsterdam Movement Sciences, Amsterdam, The Netherlands; 2. Healthy Longevity Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; 3. Centre for Healthy Longevity, @AgeSingapore, National University Health System, Singapore; 4. Department of Geriatric Medicine, Changi General Hospital, Singapore Health Services (Simei Campus), Singapore;
5. Department of Physiology, National University of Singapore (NUS), Singapore; 6. Sengkang General Hospital, Singapore; 7. SingHealth Polyclinics, Singapore.
Corresponding Author: Professor Andrea B. Maier, Centre for Healthy Longevity, National University of Singapore, 29 Medical Drive, Singapore; Email address: a.maier@nus.edu.sg
J Frailty Aging 2023;12(4)258-266
Published online July 13, 2023, http://dx.doi.org/10.14283/jfa.2023.31
Abstract
The prevalence of sarcopenia will inevitably increase as the population ages in Singapore, rendering it a growing public health concern with a significant impact on healthcare resources. This article firstly summarizes the current understanding of the epidemiology, diagnosis and management of sarcopenia, focusing on community-dwelling older individuals. Early identification is key to preventing and minimizing muscle loss. Appropriate interventions, including resistance exercise training, nutritional interventions and prehabilitation program, should be tailored to each patient. We suggest several key actions to ultimately improve awareness and overcome challenges in identifying and managing sarcopenia to improve patient outcomes. A paradigm shift where muscle health is seen as an integral component to maintaining good health with longer lifespan is needed. Education – of healthcare professionals and the public – serves as the foundation to improving awareness of muscle health and sarcopenia, and to promoting physical exercise across the age spectrum for sarcopenia prevention. The use of cost-effective evidence-based modalities (e.g., calf circumference measurement, 5-times chair stand test or bioelectric impedance assessment) enable early identification of muscle loss in routine practice. Providing subsidies for nutritional interventions (e.g., oral nutritional supplements) and exercise (e.g., ActiveSG gym membership) would encourage uptake of and adherence to interventions. Further high-quality research on interventions and their outcomes is important to determine the optimal strategy in different patient populations and to demonstrate clinical significance and value of addressing sarcopenia. Having local champions within healthcare institution would facilitate the much-needed change in healthcare culture where muscle health is a part of routine clinical practice.
Key words: Sarcopenia, muscles, aged, diagnosis, Singapore.
Introduction
Aging is associated with progressive deterioration in physiological systems (1). For the musculoskeletal system, age-related changes include skeletal muscle cell atrophy and joint degeneration, which together cause declines in lean body mass and diminished functional ability. Muscle health is defined by the presence of adequate muscle mass and muscle function, and muscle function in itself is defined as having adequate muscle strength and physical performance (2). The presence of low skeletal muscle mass, strength and/or physical performance in older individuals is defined as sarcopenia (3). Globally, sarcopenia affects approximately 10% of community-dwelling older adults (4, 5). Sarcopenia often coexists as comorbid disease (6) and with malnutrition (7, 8), and is now regarded as a precursor to frailty (9). It is a major determinant of falls and fractures (10), reduced ability to perform activities of daily living (ADLs) (11), poor health-related quality of life (HRQoL), poor post-operative outcomes (12, 13) and mortality (14–19).
The world population is aging rapidly – 1 in 6 people will be aged 60 years old or above by 2030 (20). In Singapore, about 1 in 4 people (23.8%) will be aged 65 and above by 2030 (21). Given the aging population worldwide and the inevitable increase in the prevalence of sarcopenia in the older population, skeletal muscle health will become a growing societal concern and a major source of healthcare utilization. Timely diagnosis, early intervention, and management of sarcopenia and associated risk factors are critical to improve patient outcomes. However, knowledge regarding sarcopenia is poor amongst older adults and healthcare providers (HCPs), thus accurately diagnosing and managing the condition has been underwhelming (22–24).
Early detection of sarcopenia in at-risk older adults with evidence-based targeted interventions is required to prevent and reverse progression throughout the continuum of care. In this context, the purpose of this review is to address these needs by summarizing the current understanding of the epidemiology, diagnosis, and management of sarcopenia, with a focus on community-dwelling older individuals. We also aim to provide a concerted approach to generate awareness, overcome diagnostic and interventional challenges, and to promote a holistic approach towards muscle health sustenance in Singapore.
Epidemiology of sarcopenia
The prevalence of sarcopenia varies substantially, which may be attributed, at least in part, to the measurement scale used for assessment. In a meta-analysis of Asian and European adults 60 years and older, the prevalence of sarcopenia was 11% and 9% among community-dwelling men and women, respectively (5). The meta-analysis also reported a higher prevalence of sarcopenia among hospitalized individuals (23% in men and 24% in women) and individuals living in nursing homes (51% in men and 31% in women) (5). In community-dwelling older adults (≥50 years) in Singapore, the reported prevalence of sarcopenia is 23%–46% (25–30). The prevalence may be as high as 76% in community-dwelling older adults (≥65 years) at risk of malnutrition, assessed using the Asian Working Group for Sarcopenia 2019 criteria (31).
The degree of sarcopenia depends on the presence of risk factors, including age, habitual levels of physical activity, malnutrition/malnutrition risk, and presence of comorbid diseases, such as cardiovascular disease, cognitive impairment and type 2 diabetes mellitus (T2DM) (32). Sarcopenia is highly prevalent in individuals with T2DM (31.1%) (6) and is associated with reduced energy and omega-3 fatty acid intake (33, 34) and lower participation rates in regular physical activity (35, 36). Low body mass index is also associated with sarcopenia in older patients with T2DM (37, 38). However, in a meta-analysis of individuals with T2DM of all age groups, those with T2DM showed lower muscle performance and strength, but comparable muscle mass, compared with those who are normoglycemic (39).
The association between sarcopenia, malnutrition, and frailty with poor post-operative outcomes is also increasingly recognized (12, 40, 41), with the prevalence of sarcopenia ranging between 25–42% among pre-operative individuals (18, 42–44). Furthermore, low muscle mass is associated with higher adverse post-surgical outcomes and mortality among geriatric patients (13). Sarcopenia is also associated with decreased disease-free survival and overall survival of oncology patients (18, 44, 45). These highlight the importance of identifying and counteracting sarcopenia in the older pre-operative population as well as oncology patients.
Sarcopenia definition and diagnosis
First introduced in 1988, sarcopenia was originally defined as loss of appendicular muscle mass in older individuals measured by dual energy x-ray absorptiometry (46). This definition was revised in 2010 to include both low muscle mass, muscle strength and/or physical performance, which was adopted by several consensus groups (3, 47). Subsequently, various cut-offs to define sarcopenia of aging have been employed, emphasizing the need for standardization and consideration of appropriate cut-offs for different ethnic groups. Notably, screening tools for sarcopenia, such as the SARC-F, are limited because of its low-to-moderate sensitivity (48), therefore necessitating the use of diagnostic criteria for sarcopenia without screening for identification in high-risk groups (3, 49).
The Asian Working Group for Sarcopenia 2019 (AWGS 2019) issued a consensus update on sarcopenia screening and diagnosis (3), which is utilized in Singapore. The previous sarcopenia definition was maintained, but the diagnostic algorithm, protocols and some criteria were revised (Table 1). The AWGS 2019 consensus divides diagnosis into community and hospital settings, advocating for a case-finding approach based on risk factors, looking for symptoms when relevant symptoms are reported (e.g., falling, weakness, slowness, self-reported muscle wasting, difficulties carrying out ADLs). In the consensus, the presence of low muscle strength or poor physical performance is sufficient for diagnosis of probable sarcopenia and implementation of interventions. Diagnosis of sarcopenia requires the additional assessment and confirmation of low muscle mass, while the presence of all three of low muscle strength, low physical performance, and low muscle mass indicate severe sarcopenia. Diagnostic measures of sarcopenia have to be performed by trained HCPs, with the physician being the gateway to the diagnosis of sarcopenia.
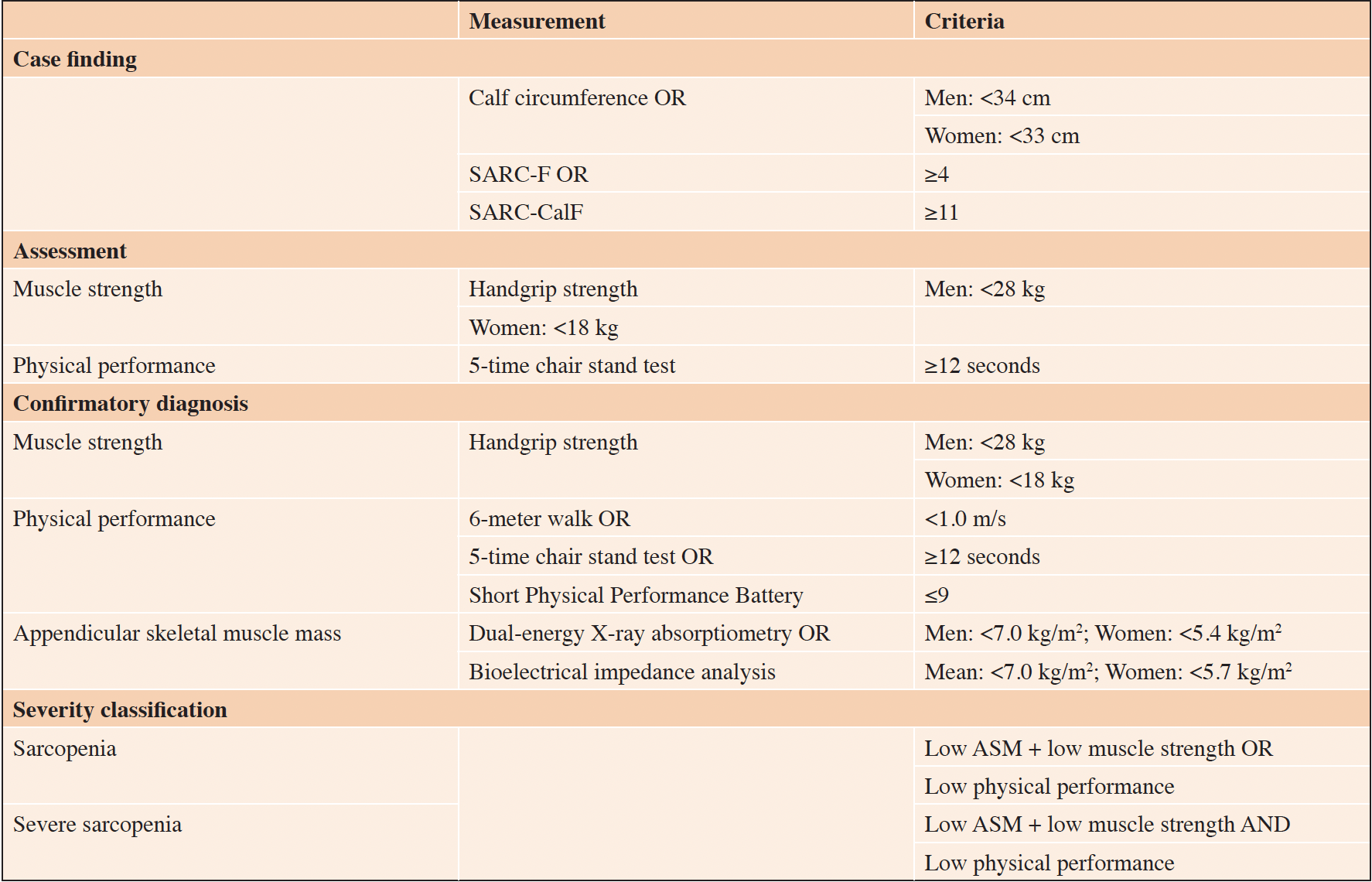
Table 1. 2019 Asian Working Group for Sarcopenia consensus for diagnosis of sarcopenia in community-dwelling adults (3)
ASM, appendicular skeletal muscle mass.
Sarcopenia interventions
Physical exercise
Regular physical exercise can modulate the biological hallmarks of aging to delay age-related chronic diseases and maintain functional capacity (50). The mechanisms responsible for the effects of lifelong exercise that positively affect muscle health may act through the promotion of anti-inflammatory pathways in skeletal muscle, largely through the release of muscle-derived myokines (51–53). Exercise, including endurance training and resistance training, has been shown to improve muscle oxidative capacity in older adults (54, 55), which in turn improves muscle function.
From a public health perspective, a higher number of steps taken by an older individual has been demonstrated to be strongly and consistently associated with better clinical outcomes, including greater muscular strength (56). There is also consensus for resistance exercise training (RET) as a key approach to manage sarcopenia (57, 58). A meta-analysis found that the intensity of RET is positively associated with the degree of muscle strength improvement in older adults (57). Progressive RET is recommended to improve muscle strength in older adults; this involves the use of free-weight or machines, where multiple- and single-joint exercises are administered, with slow-to-moderate lifting velocity for 1–3 sets/exercise, at 60–80% of 1 repetition maximum, for 8–12 repetitions with 1–3 minutes of rest in between sets, and a frequency of 2–3 days/week (59, 60). Both the American College of Sports Medicine and World Health Organization (WHO) also recommends muscle-strengthening exercises at moderate or greater intensity involving all major muscle groups on 2 or more days a week for older adults (61, 62). In addition to the multiple metabolic and physiological aerobic benefits from RET, the improvement in muscle power in itself is important as it is a predictor of functional capacity, and hence may help prevent loss or decline in functional ability (59).
Physical exercise has also been shown to benefit muscle health in individuals with T2DM, including positive effects of resistance training on lower body strength (63), and structured strength and balance training on functional status and balance confidence (64). Telerehabilitation is a useful alternative for improving physical fitness and muscle strength in older adults, particularly for those who face challenges in daily commuting (65).
Nutritional intervention
The onset and progression of sarcopenia is multifactorial and includes reduced nutrient intake and absorption (66). The impact of malnutrition on muscle mass is recognised (8), and low muscle mass is now one of the criteria for the diagnosis of malnutrition based on the Global Leadership Initiative on Malnutrition consensus criteria (67). The recently published AWGS Expert Consensus on the role of nutrition in muscle health recommends that targeted nutritional requirements should primarily be met via regular dietary patterns prior to dietary enrichment or supplementation (68). The ESPEN clinical practice guidelines recommend an energy intake of 30 kcal/kg body weight/day and a daily protein intake of at least 1 g/kg body weight for older individuals; this should be adjusted according to individual nutritional status, levels of habitual physical activity, disease state and tolerance (69). Nutritional counselling and education should also be offered by HCPs to help older individuals achieve their nutritional intake goals (68, 69). Communal or social eating can also promote regular food intake among older individuals (68).
The benefits of nutritional supplementation to manage sarcopenia in older adults have been well described. A systematic review concluded that several nutritional interventions, including amino acids, creatine, beta-hydroxy-beta-methylbutyrate (HMB) and dietary protein with amino acids supplementation, contributed to improved muscle mass in older adults (70). In Singapore, the use of HMB in community-dwelling older adults at risk of malnutrition improved nutritional outcomes and reversed risk of malnutrition, leading to significant improvement in leg strength (12.85 ± 0.22 kg vs 12.17 ± 0.22 kg at day 90) and handgrip strength (14.18 ± 0.17 kg vs 13.70 ± 0.17 kg for females at day 180) compared to control group (71). Significantly greater calf circumference (30.64 ± 0.17 cm vs 30.24 ± 0.16 cm at day 90) was also seen following HMB use among participants with low appendicular skeletal mass index (ASMI) at baseline (71). Vitamin D supplementation has been shown to have a beneficial effect on muscle strength (72). Furthermore, the AWGS Expert Consensus group recommends that vitamin D status should be assessed in patients at risk of malnutrition or sarcopenia (68). A vitamin D and leucine-enriched whey protein oral nutrition supplement (ONS) used for 13 weeks improved muscle mass and lower-extremity function in older adults with sarcopenia (66). A systematic review and meta-analysis showed that while leucine supplementation alone has no effect on muscle mass and strength, leucine-combined supplementation including with vitamin D demonstrated significant benefit for muscle strength and performance in older adults (73). As such, ONS and other specialized nutrients (e.g., HMB) can be added if nutritional requirements are still not met, or if deficiencies are identified in older adults (68).
Combined physical exercise and nutritional intervention
Combined interventions that include exercise and nutritional intervention significantly improve muscle health. Combined progressive RET and dietary protein intervention for 12 weeks was effective in improving lean body mass and muscle strength in community-dwelling older adults (74). Both protein supplementation and RET resulted in greater muscle mass (i.e., appendicular muscle mass and fat-free mass) and muscle strength (i.e., handgrip strength, knee extension strength and leg press strength) in older adults (75, 76). Combined vitamin D supplementation and RET also led to significant improvement in muscle strength of the lower limb when compared with the effects from each intervention alone (77). In older adults with T2DM, a multimodal training intervention, comprising of personalized RET, nutritional counselling and general health counselling demonstrated improvements in muscle health (78).
Guidelines have recommended such combined approaches for the management and prevention of sarcopenia, including the recently published Singapore Clinical Practice Guidelines for Sarcopenia (49, 68, 69). It is however important to ensure that older adults who exercise have adequate energy and protein intake to maintain body weight and maintain/improve muscle mass (69). The AWGS Expert Consensus recommends a combined approach using nutrition and resistance exercise interventions in a tailored and individualized manner for best outcomes (68). Recommended follow-up measures to assess response to interventions include nutritional, anthropometric, muscle health, functional, and HRQoL outcomes (68).
Prehabilitation for patients with sarcopenia undergoing surgery
Major surgical procedures can substantially reduce physiological and functional capacity, and the associated recovery process can lead to muscle atrophy and deterioration (79). As such, addressing sarcopenia prior to surgery through prehabilitation may help prepare individuals for their surgery by improving the functional capacity to tolerate stress from surgery thereby reducing complications and improving recovery (79). Studies assessing benefits of exercise and nutritional interventions are typically conducted over a long duration (≥ 10 weeks) and yet, the lead up to major surgery is often only a few weeks long. There is thus a need for further study evaluating interventions that are impactful within a short intervention duration. The HEROS study (NCT05344313) is an ongoing study assessing the effect of ONS with HMB and 2–4 weeks RET prehabilitation on muscle quality in patients with sarcopenia undergoing surgery in Singapore; the outcome of this study will provide data on the optimal type and duration of intervention for this patient population.
Prehabilitation involves a multimodal approach encompassing physical exercises, nutritional optimization and psychological support (79). Prehabilitation physical exercises aim to improve aerobic capacity and develop lean muscle mass, while nutritional optimization aims to correct pre-operative malnutrition and support exercise training (80). Psychological support during prehabilitation is important to improve anxiety and depression, and to help patients develop self-efficacy for prehabilitation and surgery (80). Data suggests the benefit of prehabilitation in older patients undergoing surgery (81, 82). Prehabilitation is associated with positive surgical, oncological, functional and patient-reported outcomes and improved healthcare cost savings (83, 84). In Singapore, a surgical prehabilitation program for older adults has been successfully implemented in Sengkang General Hospital. The program includes dietitian assessment and nutritional supplementation; physiotherapy assessment, RET and deep breathing exercise; and geriatric assessment – the program is completed within 2–4 weeks leading up to surgery.
Expert commentary: a call to action
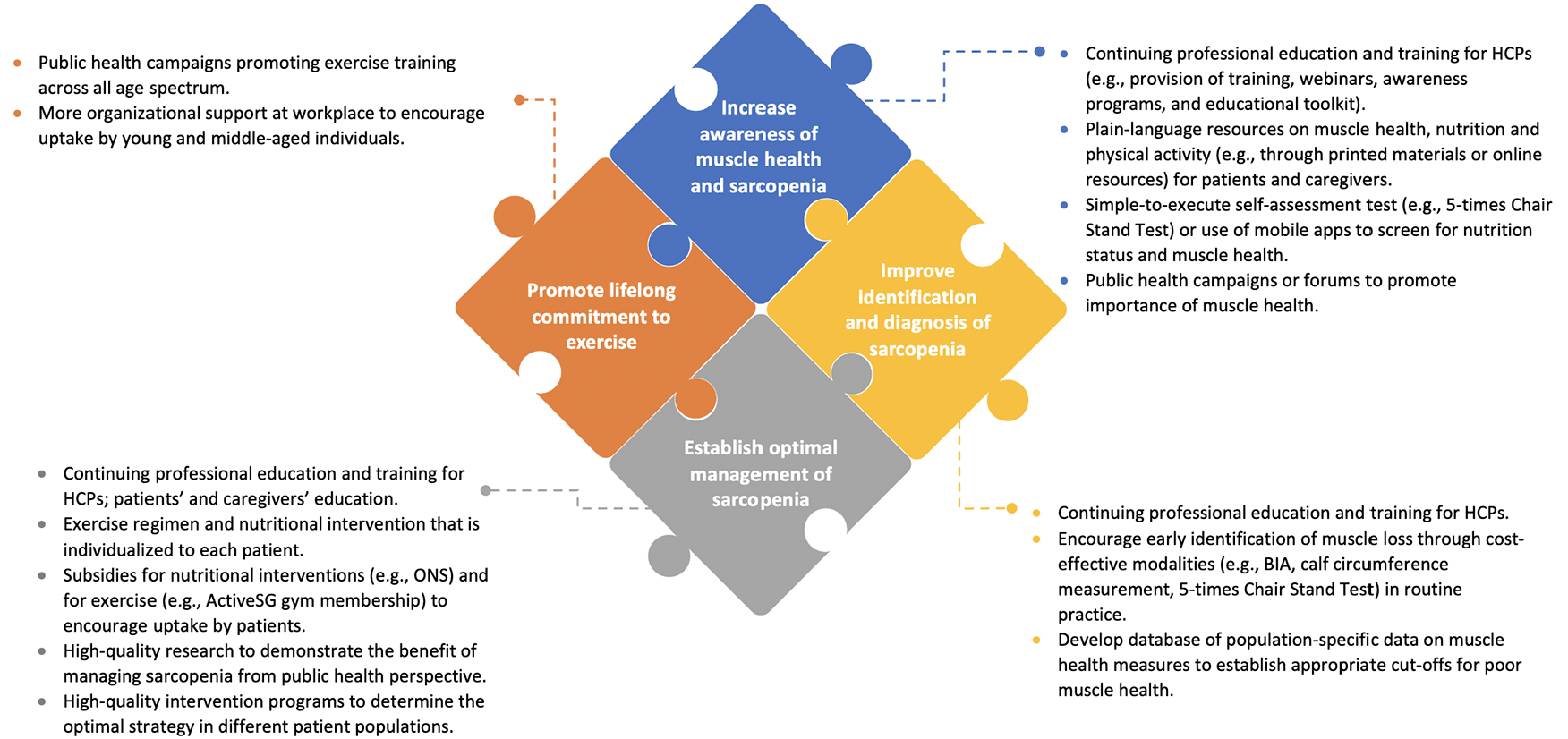
To optimize muscle health in older adults, improvements in awareness, diagnosis and management across the continuum of care is imperative. A paradigm shift is required where muscle health is seen as critical to good health, not only in avoiding adverse short term outcomes, but also in enabling and maintaining independent living for as long as possible and leading to high quality and meaningful life. In this context, we have provided several key priorities and potential actions for adoption within routine clinical care, with the ultimate goal of improving outcomes for patients.
Increasing awareness of the adverse clinical impact of sarcopenia
Key priorities for adoption of sarcopenia diagnosis and management initially require the recognition by both HCPs and the public of the importance of muscle health and the recognition of the impact sarcopenia can have. However, the concept of sarcopenia is not well recognized nor understood by either (85). Only half of HCPs across five continents involved in the care of older adults with musculoskeletal conditions measure at least one of the three domains of muscle mass, muscle strength, or physical performance in their clinical practice (86). Issues identified included a lack of standardization in the assessment tool and protocol, leading to difficulty in implementation and comparing results. In a study of Australian and New Zealand and Dutch HCPs, barriers to identification of sarcopenia included lack of awareness and knowledge, with engaging in continuous professional development to acquire up-to-date knowledge suggested as a means to overcome this issue (22, 23). Limitations in awareness of the health impact of sarcopenia by HCPs may be addressed, at least in part, through professional education and training. For instance, provision of training, webinars, awareness programs, and ‘toolkits’ comprising scientific information and illustrations for HCPs should be prioritized to explain assessment of muscle strength, muscle mass, and physical performance, and in prevention and management, as well as the differences in international guidelines and what is required for the local population across the continuum of care.
Patient awareness and knowledge is of utmost importance to improve muscle health and prevent sarcopenia, as ensuring adequate nutrition intake and regular RET requires patient active efforts. However, patient’s lack of knowledge and poor/different understanding of sarcopenia (24, 85, 87) necessitates improving their awareness regarding the importance of muscle health, which can be done through health promotion advertisements, campaigns, and public forums. Promoting awareness and self-assessment of muscle health among older patients can be explored using simple-to-execute tests such as the 5-times Chair Stand Test (47), or through the use of mobile apps to screen for nutritional status and muscle health (88). HCPs should also be relied upon to implement educational strategies for patients, families and caregivers; this may include verbal/written advice and plain-language resources on muscle health, nutrition and physical activity (2). Furthermore, patients can be encouraged to ask questions to their HCPs.
With limited resources, it may be difficult to invest in measuring muscle health. However, because muscle health has multiple ramifications including falls prevention, reduction in disability and mortality from falls-related trauma, and decreases in disability and dependency, investing in measuring muscle health will ultimately lead to reduced healthcare spending (89, 90) and higher HRQoL (91). This benefit of measuring muscle health should be supported by high-quality population health and health economics studies to provide an evidence base supporting the projected improvements in population health and reduction in healthcare costs. This would require an increase in the scope of research, such as grants for capability developments and validation, and through higher hospital management buy-in. Because muscle health also impacts bone health, cardiovascular health, and cognitive wellbeing (92), incorporating muscle health as part of the current public health campaigns in these areas could minimize the costs of establishing a public health campaign highlighting muscle health (47).
Improving identification and diagnosis of sarcopenia
Early identification of patients at risk of sarcopenia is a key priority, to enable prevention and management strategies to be implemented in a timely manner and to improve outcomes (3). Although computed tomography (CT) scanning for diagnosis of sarcopenia may be costly, involves radiation, or may not be easily accessible, there are surrogate measures and other modalities which are cost effective and can be deployed in the clinical setting such as use of Bioelectric Impedance Assessment (BIA), calf circumference measurement, and use of 5-times Chair Stand Test for lower limb strength (93) or as a surrogate measure of gait speed (94). These are now also supported by consensus guidelines (3). More precise and clinically predictive tests for muscle mass such as the deuterated creatine dilution test may become more accessible in the near future and further enhance our ability to diagnose poor muscle health (2). Training for HCPs in sarcopenia diagnosis should also include understanding differences in cut-offs or measurement criteria relevant to the local population based on available diagnostic tools. Newer technologies, such as ultrasound, need to be tested regarding their feasibility for sarcopenia diagnosis (95).
Population-specific data from a large and continuous database of muscle health measures (e.g., grip strength, knee extensor strength, calf circumference, gait speed and 5-times Chair Stand Test) in younger adults would also help establish appropriate cut-offs for poor muscle health in older adults.
Establishing the optimal management of sarcopenia
The pillars of improving muscle health in older individuals are nutritional interventions and RET, delivered in an integrated and tailored manner (47, 49, 68, 96). Although maintenance or improvement in both muscle mass and function should be the ultimate goal of sarcopenia management, all interventions must at least demonstrate improvements in nutritional status and muscle mass. Studies have shown that individuals with an energy deficient diet have a measurable reduction in their muscle protein synthesis rate (97). In a glycogen-depleted state, studies have also shown a doubling rate of muscle protein catabolism during increased physical activity (98). As such, identifying and treating any underlying concomitant protein and energy malnutrition is necessary before putting older adults into RET regimen. Once good nutritional health is achieved and muscle strength returned to normative values, increased muscle mass and quality can be additional outcome measures.
Management strategies should be tailored to each patient, according to underlying conditions (medical, physical and psychological) and individual circumstances that may act as barriers to the uptake and adherence to nutritional interventions and exercise regimen. Subsidies for nutritional interventions (e.g., ONS) and for exercise (e.g., ActiveSG gym membership) could help encourage uptake by older patients. Specific considerations regarding RET in older adults are also required, as they have different needs and require more individualization in terms of the training regimen as compared to young adults. Older adults may also have more intense delayed onset muscle soreness (DOMS) post-RET (99) and hence may require pre-training counselling and post-training mitigation strategies, such as allowing more recovery time and use of massage for relief (100, 101).
As with improving awareness and diagnosis of sarcopenia in older adults, resources for provision of evidence-based interventions for muscle health in older adults are also inadequate. Although global data is emerging of the substantial downstream benefits if muscle health and nutrition are managed, further good quality research is needed to demonstrate that management of sarcopenia makes a difference, including in a value-driven healthcare system such as that in Singapore. Such provision of evidence-based data would benefit from the sharing of resources and protocols, as well as collaboration. Similarly, high quality, prospective outcome measures built into intervention programs on nutritional, muscle health, clinical, functional outcomes, and HRQoL are required, including among particular patient subgroups, such as those with T2DM and post-operative patients, to fine tune the interventions, leading to a virtuous circle.
Promoting lifelong commitment to physical activity for sarcopenia prevention
A commitment to muscle health requires a lifelong dedication to physical activity participation. Considering the age spectrum through which sarcopenia presents, preventive efforts need to be enhanced to slow or prevent sarcopenia in the middle-aged, or even among younger populations, particularly if they have low skeletal muscle mass to begin with. With the current emphasis on physical activity as the cornerstone of health by the WHO (61, 102), the time is ripe to encourage and facilitate lifelong commitment to physical activity, not only in older adults but throughout the whole population starting from childhood into adulthood.
Physical activity participation rates in the older population (> 60 years) are low in Singapore. In a 2015 Sports and Physical Activity survey conducted in ~7500 households, it was reported that about 58% of Singaporeans aged > 60 years engaged in less than 3 days per week of leisure time physical activity or exercise (103), which is less than the minimum guidelines recommended by the WHO (150 minutes of moderate-intensity physical activity per week) (61). A cross-sectional study of multi-ethnic Asian aged ≥60 years in a Singapore public primary healthcare centre found that the physical activity of older adults decreased with increasing age and those with employment were twice as likely to have sedentary behaviour (104). More worryingly, ~76% of Singaporeans between the ages of 40-59 years also did not meet the minimum guideline recommended by the WHO (103). These statistics indicate that most middle-aged and older Singaporeans are not physically active – these are alarming because they reflect a relatively uninformed population regarding healthy lifestyle practices. The term ‘physical activity’ is broad and includes recreational, occupational and travel activities. Exercise training is a subset of physical activity that aims to improve cardiorespiratory and muscular fitness beyond what can be achieved with routine physical activity. The numbers for regular participation in exercise training focusing on improving cardiorespiratory and muscular fitness indicate poor uptake (105). Thus, there needs to be a greater emphasis on improving public awareness and uptake of exercise training. In other words, the quality of habitual physical activity matters – this should be part of the education of the general public and HCPs. More should be done at the policy level to improve organizational support at the workplace to enhance uptake amongst young and middle-aged individuals as the nation embraces healthier Singapore.
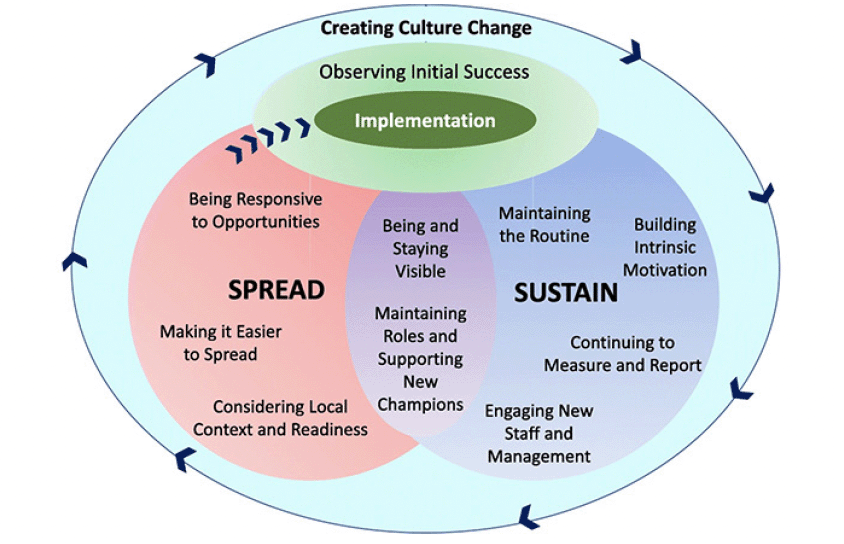
Creating and sustaining culture change to strengthen muscle health importance
There is an urgent need for a change in the healthcare culture to incorporate muscle health as part of routine clinical practice. A framework such as ‘Sustain and Spread’ (Figure 1) can be used to steer organizational culture change (106). Local champions within healthcare institutions would help enable, encourage and acknowledge the efforts in screening, assessment and management of sarcopenia so that these are sustained and embedded in daily practice. In this framework, once there is initial implementation success, several strategies are employed to sustain and spread the successful changes thereby leading to an overall culture change (106).
Reproduced from Figure 1, Laur C et al., published under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) (106).
Conclusion
With the rapidly aging population in Singapore, muscle health is soon becoming a growing public health concern. Early identification of sarcopenia in at-risk older individuals along with evidence-based interventions are key to prevent and reverse the progression of sarcopenia. Therefore, urgent actions to increase awareness of muscle health and improve the diagnosis and management of sarcopenia across the continuum of care are imperative (Figure 2). To achieve these, there is a need for active dialogues and collaboration among various stakeholders (HCPs, professional societies, patients and their support networks, and policy makers).
Abbreviations: BIA, bioelectric impedance assessment; HCPs, healthcare providers; ONS, oral nutrition supplement.
Acknowledgment: Medical writing support for this manuscript was provided by Tricia Newell, PhD, from In Vivo Communications Pte Ltd.
Funding: The development of this manuscript was supported by Abbott Laboratories (Singapore) Pte Ltd., which was strictly limited to editorial support provided by the medical writer (see acknowledgements). All authors did not receive any honorarium from Abbott Laboratories (Singapore) Pte Ltd. for the preparation of this manuscript. Abbott Laboratories (Singapore) Pte Ltd. had no role in the preparation or approval of this manuscript.
Conflict of interest disclosures: AB Maier has received consultation fees from Nutricia and Abbott Nutrition. STH Chew has received grant co-funding, travel grant and honoraria from Abbott Nutrition. NC Tan is the site principal investigator of a clinical trial sponsored by Abbott Nutrition. FHX Koh and J Goh have nothing to declare.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Bautmans I, Knoop V, Thiyagarajan JA, Maier AB, Beard JR, Freiberger E, et al. WHO working definition of vitality capacity for healthy longevity monitoring. Lancet Healthy Longev 2022;3(11):e789–e796. doi: 10.1016/S2666-7568(22)00200-8.
2. Prado CM, Landi F, Chew STH, Atherton PJ, Molinger J, Ruck T, et al. Advances in muscle health and nutrition: a toolkit for healthcare professionals. Clin Nutr 2022;41(10):2244–63. doi: 10.1016/j.clnu.2022.07.041.
3. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-7.e2.C doi: 10.1016/j.jamda.2019.12.012.
4. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord 2017;16:21. doi: 10.1186/s40200-017-0302-x.
5. Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging 2020;24:83–90. doi: 10.1007/s12603-019-1267-x.
6. Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp Gerontol 2020;131:110801. doi: 10.1016/j.exger.2019.110801.
7. Ligthart-Melis GC, Luiking YC, Kakourou A, Cerderholm T, Maier AB, de van der Schueren MAE. Frailty, sarcopenia, and malnutrition Frequently (Co-)occur in Hospitalized Older Adults: A Systematic Review and Meta-analysis. J Am Med Dir Assoc 2020;21:1216-28. doi: 10.1016/j.jamda.2020.03.006.
8. Landi F, Camprubi-Robles M, Bear DE, Cederholm T, Malafarina V, Welch AA, et al. Muscle loss: The new malnutrition challenge in clinical practice. Clin Nutr 2019;38:2113-20. doi: 10.1016/j.clnu.2018.11.021.
9. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006.
10. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2019;10:485-500. doi: 10.1002/jcsm.12411.
11. Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle 2020;11:3-25. doi: 10.1002/jcsm.12502.
12. Prado CM, Purcell SA, Alish C, Pereira SL, Deutz NE, Heyland DK, et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med 2018;50:675-93. doi: 10.1080/07853890.2018.1511918.
13. Hossain M, Yu D, Bikdeli B, Yu S. Sarcopenia and Adverse Post-Surgical Outcomes in Geriatric Patients: A Scoping Review. J Frailty Aging 2021;10:63-9. doi: 10.14283/jfa.2020.27.
14. Xu J, Reijnierse EM, Pacifico J, Wan CS, Maier AB. Sarcopenia is associated with 3-month and 1-year mortality in geriatric rehabilitation inpatients: RESORT. Age Ageing 2021;50:2147-56. doi: 10.1093/ageing/afab134.
15. Xu J, Wan CS, Ktoris K, Reijnierse EM, Maier AB. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2022;68:361-76. doi: 10.1159/000517099.
16. Scheerman K, Meskers CGM, Verlaan S, Maier AB. Sarcopenia, Low Handgrip Strength, and Low Absolute Muscle Mass Predict Long-Term Mortality in Older Hospitalized Patients: An Observational Inception Cohort Study. J Am Med Dir Assoc 2021;22:816-20.e2. doi: 10.1016/j.jamda.2020.
17. Pacifico J, Reijnierse EM, Lim WK, Maier AB. The Association between Sarcopenia as a Comorbid Disease and Incidence of Institutionalisation and Mortality in Geriatric Rehabilitation Inpatients: REStORing health of acutely unwell adulTs (RESORT). Gerontology 2022;68:498-508. doi: 10.1159/000517461.
18. Trejo-Avila M, Bozada-Gutierrez K, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:1077-96. doi: 10.1007/s00384-021-03839-4.
19. Hu X, Zhang L, Wang H, Hao Q, Dong B, Yang M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep 2017;7:3171. doi: 10.1038/s41598-017-03388-3.
20. World Health Organization. 2022. Ageing and Health. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed 16 January 2023.
21. National Population and Talent Division. Population Trends 2022. Available from: https://www.population.gov.sg/our-population/population-trends/overview/. Accessed 16 January 2023.
22. Reijnierse EM, de van der Schueren MAE, Trappenburg MC, Doves M, Meskers CGM, Maier AB. Lack of knowledge and availability of diagnostic equipment could hinder the diagnosis of sarcopenia and its management. PLoS One 2017;12:e0185837. doi: 10.1371/journal.pone.0185837.
23. Yeung SSY, Reijnierse EM, Trappenburg MC, Merskers CGM, Maier AB. Current knowledge and practice of Australian and New Zealand health-care professionals in sarcopenia diagnosis and treatment: Time to move forward! Australas J Ageing 2020;39:e185-e93. doi: 10.1111/ajag.12730.
24. Van Ancum JM, Meskers CGM, Reijnierse EM, Yeung SSY, Jonkman NH, Trappenburg MC, et al. Lack of Knowledge Contrasts the Willingness to Counteract Sarcopenia Among Community-Dwelling Adults. J Aging Health 2020;32:787-94. doi: 10.1177/0898264319852840.
25. Fung FY, Koh YLE, Malhotra R, Ostbye T, Lee PY, Ghazali SS, et al. Prevalence of and factors associated with sarcopenia among multi-ethnic ambulatory older Asians with type 2 diabetes mellitus in a primary care setting. BMC Geriatr 2019;19:122. doi: 10.1186/s12877-019-1137-8.
26. Keng BMH, Gao F, Teo LLY, Lim WS, Tan RS, Ruan W, et al. Associations between skeletal muscle and myocardium in aging: a syndrome of «Cardio-Sarcopenia»? J Am Geriatr Soc 2019;67:2568-73. doi: 10.1111/jgs.16132.
27. Lamourex EL, et al. The combination of sarcopenia and frailty increases the odds of cognitive impairment in elderly Singaporeans. Poster 142. The Journal of Frailty & Aging 2020;9 (Suppl 1):46-179. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7223455/. Accessed October 9, 2022. doi: 10.14283/jfa.2020.9.
28. Lu Y, Karagounis LG, Ng TP, Carre C, Narang V, Wong G, et al. Systemic and Metabolic Signature of Sarcopenia in Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci 2020;75:309-17. doi: 10.1093/gerona/glz001.
29. Pang BWJ, Wee SL, Lau LK, Jabbar KA, Seah WT, Ng DHM, et al. Prevalence and associated factors of sarcopenia in Singaporean adults – The Yishun Study. J Am Med Dir Assoc 2021;22:885.e1-.e10. doi: 10.1016/j.jamda.2020.05.029.
30. Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr) 2015;37:121. doi: 10.1007/s11357-015-9860-3.
31. Chew STH, Tey SL, Yalawar M, Liu Z, Baggs G, How CH, et al. Prevalence and associated factors of sarcopenia in community-dwelling older adults at risk of malnutrition. BMC Geriatr. 2022;22(1):997. doi: 10.1186/s12877-022-03704-1.
32. Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F, et al. Associated factors of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients 2021;13(12):4291. doi: 10.3390/nu13124291.
33. Okamura T, Miki A, Hashimoto Y, Kaji A, Sakai R, Osaka T, et al. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J Diabetes 2019;11:477-83. doi: 10.1111/1753-0407.12874.
34. Okamura T, Hashimoto Y, Miki A, Kaji A, Sakai R, Iwai K, et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: a cross-sectional study of KAMOGAWA-DM cohort study. J Clin Biochem Nutr 2020;66:233-7. doi: 10.3164/jcbn.19-85.
35. Cui M, Gang X, Wang G, Xiao X, Li Z, Jiang Z, et al. A cross-sectional study: Associations between sarcopenia and clinical characteristics of patients with type 2 diabetes. Medicine (Baltimore) 2020;99:e18708. doi: 10.1097/MD.0000000000018708.
36. De Freitas MM, de Oliveira VLP, Grassi T, Valduga K, Miller MEP, Schuchmann RA, et al. Difference in sarcopenia prevalence and associated factors according to 2010 and 2018 European consensus (EWGSOP) in elderly patients with type 2 diabetes mellitus. Exp Gerontol 2020;132:110835. doi: 10.1016/j.exger.2020.110835.
37. Fukuoka Y, Narita T, Fujita H, Morii T, Sato T, Sassa MH, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig 2019;10:322-30. doi: 10.1111/jdi.12908.
38. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993-7. doi: 10.2337/dc09-0264.
39. Anagnostis P, Gkekas NH, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif Tissue Int 2020;107:453-63. doi: 10.1007/s00223-020-00742-y.
40. Ng ZQ, Cohen R, Misur P, Weber DG. Poorer outcomes associated with sarcopenia following emergency laparotomy: a systematic review and meta-analysis. ANZ J Surg 2022;92(12):3145–3153. doi:10.1111/ans.17641.
41. Maeda K, Saiki Y. Reconsideration of frailty in relation to surgical indication. Gen Thorac Cardiovasc Surg 2018;66:201-13. doi: 10.1007/s11748-017-0869-7.
42. Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and inflammatory bowel diasease: a systematic review. Inflamm Bowel Dis 2019;25(1):67–73. doi: 10.1093/ibd/izy212.
43. Okamura H, Kimura N, Mieno M, Yuri K, Yamaguchi A. Preoperative sarcopenia is associated with late mortality after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg 2020;58(1):121–9. doi: 10.1093/ejcts/ezz378.
44. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore) 2016;95:e3164. doi: 10.1097/MD.0000000000003164.
45. Ubach J, Ziemons J, Minis-Rutten IJG, Kruitwagen RFPM, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2019;10(6):1165–74. doi: 10.1002/jcsm.12468.
46. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle 2019;10:956-61. doi: 10.1002/jcsm.12483.
47. Chew STH, Kayambu G, Lew CCH, Ng TP, Ong FY, Tan J, et al. Singapore multidisciplinary consensus recommendations on muscle health in older adults: assessment and multimodal targeted intervention across the continuum of care. BMC Geriatr 2021;21:314. doi: 10.1186/s12877-021-02240-8.
48. Voelker SN, Michalopoulos N, Maier AB, Reijnierse EM. Reliability and Concurrent Validity of the SARC-F and Its Modified Versions: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc 2021;22:1864-76.e16. doi: 10.1016/j.jamda.2021.05.011.
49. Lim WS, Cheong CY, Lim JP, Tan MMY, Chia JQ, Malik NA, et al. Singapore clinical practice guidelines for sarcopenia: screening, diagnosis, management and prevention. J Frailty Aging 2022;11(4):348–69. doi: 10.14283/jfa.2022.59.
50. Goh J, Wong E, Soh J, Maier AB, Kennedy BK. Targeting the molecular & cellular pillars of human aging with exercise. FEBS J 2021;290(3):649–668. doi:10.1111/febs.16337.
51. Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 2020;128:87-99. doi: 10.1152/japplphysiol.00495.2019.
52. Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 2018;15:731-43. doi: 10.1038/s41569-018-0065-1.
53. Oudbier SJ, Goh J, Looijaard SMLM, Reijnierse EM, Meskers CGM, Maier AB. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J Gerontol A Biol Sci Med Sci 2022;77(10):1959-1968. doi: 10.1093/gerona/glac121.
54. Conley KE, Jubrias SA, Cress ME, Esselman PC. Elevated energy coupling and aerobic capacity improves exercise performance in endurance-trained elderly subjects. Exp Physiol 2013;98(4):899-907. doi: 10.1113/expphysiol.2012.069633.
55. Irving BA, Lanza IR, Henderson GC, Rao RR, Spiegelman BM, Nair KS. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab 2015;100(4):1654–63. doi: 10.1210/jc.2014-3081.
56. Ramsey KA, Meskers CGM, Maier AB. Every step counts: synthesising reviews associating objectively measured physical activity and sedentary behaviour with clinical outcomes in community-dwelling older adults. The Lancet Healthy Longevity 2021;2:e764-e72. doi: 10.1016/S2666-7568(21)00203-8.
57. Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226-37. doi: 10.1016/j.arr.2010.03.004.
58. Montero-Fernández N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013;49:131-43.
59. Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25(7):824-853. doi: 10.1007/s12603-021-1665-8.
60. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41(3):687-708. doi: 10.1249/MSS.0b013e3181915670.
61. World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization; 20202020. Available from: https://www.who.int/publications/i/item/9789240015128. Accessed October 9, 2022.
62. American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41(7):1510-30. doi: 10.1249/MSS.0b013e3181a0c95c.
63. Seyedizadeh SH, Cheragh-Birjandi S, Nia MRH. The Effects of Combined Exercise Training (Resistance-Aerobic) on Serum Kinesin and Physical Function in Type 2 Diabetes Patients with Diabetic Peripheral Neuropathy (Randomized Controlled Trials). J Diabetes Res 2020;2020:6978128. doi: 10.1155/2020/6978128.
64. Venkataraman K, Tai BC, Khoo EYH, Tavintharan S, Chandran K, Hwang SW, et al. Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: a randomised controlled trial. Diabetologia 2019;62:2200-10. doi: 10.1007/s00125-019-04979-7.
65. Duruturk N, Özköslü MA. Effect of tele-rehabilitation on glucose control, exercise capacity, physical fitness, muscle strength and psychosocial status in patients with type 2 diabetes: A double blind randomized controlled trial. Prim Care Diabetes 2019;13:542-8. doi: 10.1016/j.pcd.2019.03.007.
66. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015;16:740-7. doi: 10.1016/j.jamda.2015.05.021.
67. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al; GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1-9. doi: 10.1016/j.clnu.2018.08.002.
68. Chen LK, Arai H, Assantachai P, Akishita M, Chew STH, Dumlao LC, et al. Roles of nutrition in muscle health of community-dwelling older adults: evidence-based expert consensus from Asian Working Group for Sarcopenia. J Cachexia Sarcopenia Muscle 2022;13:1653-72. doi: 10.1002/jcsm.12981.
69. Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Hooper L, Kiesswetter E, Maggio M, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr 2022;41:958-89. doi: 10.1016/j.clnu.2022.01.024.
70. Martin-Cantero A, Reijnierse EM, Gill BMT, Maier AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta-analysis. Nutr Rev 2021;79:315-30. doi: 10.1093/nutrit/nuaa064.
71. Chew STH, Tan NC, Cheong M, Oliver J, Baggs G, Choe Y, et al. Impact of specialized oral nutritional supplement on clinical, nutritional, and functional outcomes: A randomized, placebo-controlled trial in community-dwelling older adults at risk of malnutrition. Clin Nutr 2021;40:1879-92. doi: 10.1016/j.clnu.2020.10.015.
72. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742.
73. Guo Y, Fu X, Hu Q, Chen L, Zuo H. The effect of leucine supplementation on sarcopenia-related measures in older adults: a systematic review and meta-analysis of 17 randomized controlled trials. Front Nutr 2022;9:929891. doi: 10.3389/fnut.2022.929891.
74. Van Dongen EJI, Haveman-Nies A, Doets EL, Dorhout BG, de Groot LCPGM. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in Practice. J Am Med Dir Assoc 2020;21(8):1065-1072.e3. doi: 10.1016/j.jamda.2019.11.026.
75. Kirwan RP, Mazidi M, Garcia CR, Lane KE, Jafari A, Butler T, et al. Protein interventions augment the effect of resistance exercise on appendicular lean mass and handgrip strength in older adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2022;115:897-913. doi: 10.1093/ajcn/nqab355.
76. Hou L, Lei Y, Li X, Huo C, Jia X, Yang J, et al. Effect of Protein Supplementation Combined with Resistance Training on Muscle Mass, Strength and Function in the Elderly: A Systematic Review and Meta-Analysis. J Nutr Health Aging 2019;23:451-8. doi: 10.1007/s12603-019-1181-2.
77. Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D(3) supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open 2017;7:e014619. doi: 10.1136/bmjopen-2016-014619.
78. Rodriguez-Mañas L, Laosa O, Vellas B, Paolisso G, Topinkova E, Oliva-Moreno J, et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle 2019;10:721-33. doi: 10.1002/jcsm.12432.
79. Koh FH, Chua JM, Tan JL, Foo FJ, Tan WJ, Sivarajah SS, et al. Paradigm shift in gastrointestinal surgery – combating sarcopenia with prehabilitation: Multimodal review of clinical and scientific data. World J Gastrointest Surg 2021;13:734-55. doi: 10.4240/wjgs.v13.i8.734.
80. Durrand J, Singh SJ, Danjoux G. Prehabilitation. Clin Med (Lond) 2019;19(6):458-464. doi: 10.7861/clinmed.2019-0257.
81. Janssen TL, Steyerberg EW, Langenberg JCM, van Hoof – de Lepper CCHA, Wielders D, Seerden TCJ, et al. Multimodal prehabilitation to reduce the incidence of delirium and other adverse events in elderly patients undergoing elective major abdominal surgery: an uncontrolled before-and-after study. PLoS One 2019;14:e0218152. doi: 10.1371/journal.pone.0218152.
82. Barberan-Garcia A, Ubre M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalized prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50-56. doi: 10.1097/SLA.0000000000002293.
83. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A Systematic Review and Meta-analysis of Physical Exercise Prehabilitation in Major Abdominal Surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg 2020;24:1375-85. doi: 10.1007/s11605-019-04287-w.
84. Koh FH, Loh CH, Tan WJ, Ho LML, Yen D, Chua JMW, et al. Structured presurgery prehabilitation for aged patients undergoing elective surgery significantly improves surgical outcomes and reduces cost: A nonrandomized sequential comparative prospective cohort study. Nutr Clin Pract 2022;37:645-53. doi: 10.1002/ncp.10787.
85. Dent E, Woo J, Scott D, Hoogendijk EO. Toward the recognition and management of sarcopenia in routine clinical care. Nature Aging 2021;1:982-90. doi: 10.1038/s43587-021-00136-1.
86. Bruyère O, Beaudart C, Reginster JY, Buckinx F, Schoene D, Vasant H, et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. European Geriatric Medicine 2016;7:243-6. doi: 10.1016/j.eurger.2015.12.009
87. Zanker J, Sim M, Anderson K, Balogun S, Brennan-Olsen SL, Dent E, et al. The Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) sarcopenia diagnosis and management task force: Findings from the consumer expert Delphi process. Australas J Ageing 2022;doi:10.1111/ajag.13164.
88. Krznarić Ž, Bender DV, Laviano A, Cuerda C, Landi F, Monteiroet R, et al. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr. 2020;39(7):1983-1987. doi: 10.1016/j.clnu.2020.05.006.
89. Bruyère O, Beaudart C, Ethgen O, Reginster JY, Locquet M. The health economics burden of sarcopenia: a systematic review. Maturitas 2019;119:61-69. doi: 10.1016/j.maturitas.2018.11.003.
90. Norman K, Otten L. Financial impact of sarcopenia or low muscle mass – a short review. Clin Nutr 2019;38(4):1489–95. doi: 10.1016/j.clnu.2018.09.026.
91. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48(1):16–31. doi: 10.1093/ageing/afy169.
92. Hillsdon M, Foster C. What are the health benefits of muscle and bone strengthening and balance activities across life stages and specific health outcomes? J Frailty Sarcopenia Falls 2018;3(2):66–73. doi: 10.22540/JFSF-03-066.
93. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113-9. doi: 10.1080/02701367.1999.10608028.
94. Nishimura T, Arima K, Okabe T, Mizukami S, Tomita Y, Kanagae M, et al. Usefulness of chair stand time as a surrogate of gait speed in diagnosing sarcopenia. Geriatr Gerontol Int. 2017;17(4):668-669. doi: 10.1111/ggi.12882.
95. Stringer HJ, Wilson D. The Role of Ultrasound as a Diagnostic Tool for Sarcopenia. J Frailty Aging 2018;7:258-61. doi: 10.14283/jfa.2018.24.
96. Daly RM, Iuliano S, Ffye JJ, Scott D, Kirk B, Thompson MQ, et al. Screening, Diagnosis and Management of Sarcopenia and Frailty in Hospitalized Older Adults: Recommendations from the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) Expert Working Group. J Nutr Health Aging. 2022;26(6):637-651. doi: 10.1007/s12603-022-1801-0.
97. Areta JL, Burke ML, Camera DM, West DWD, Crawshay S, Moore DR, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab. 2014;306(8):E989-97. doi: 10.1152/ajpendo.00590.2013.
98. Gillen JB, West DWD, Williamson EP, Fung HJW, Moore DR. Low-Carbohydrate Training Increases Protein Requirements of Endurance Athletes. Med Sci Sports Exerc. 2019;51(11):2294-2301. doi: 10.1249/MSS.0000000000002036.
99. Hayes EJ, Stevenson E, Sayer AA, Granic A, Hurst C. Recovery from resistance exercise in older adults: a protocol for a scoping review. BMJ Open Sport Exerc Med 2022;8(1):e001229. doi: 10.1136/bmjsem-2021-001229.
100. Guo J, Li L, Gong Y, Zhu R, Xu J, Zou J, et al. Massage Alleviates Delayed Onset Muscle Soreness after Strenuous Exercise: A Systematic Review and Meta-Analysis. Front Physiol. 2017;8:747. doi: 10.3389/fphys.2017.00747.
101. Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness : treatment strategies and performance factors. Sports Med. 2003;33(2):145-164. doi: 10.2165/00007256-200333020-00005.
102. UN Decade of Healthy Ageing 2021-2030. 2021. Available from: https://www.who.int/initiatives/decade-of-healthy-ageing. Accessed October 9, 2022
103. Marique P. Sports Index Participation Trends 2015. Market Insights & Consumer Analytics (Sport Singapore); 2016. Available from: https://www.sportsingapore.gov.sg/-/media/SSC/Corporate/Files/About/Publications/Sports-Index-2015.pdf. Accessed 16 January 2023.
104. Ng LP, Koh LYE, Tan NC. Physical activity and sedentary behaviour of ambulatory older adults in a developed Asian community: a cross-sectional study. Singapore Med J 2020;61(5):266–71. doi: 10.11622/smedj.2020022.
105. Ministry of Health Singapore. National Population Health Survey 2020 (Household Interview and Health Examination). Available from: https://www.moh.gov.sg/docs/librariesprovider5/default-document-library/nphs-2020-survey-report.pdf. Accessed October 9, 2022.
106. Laur C, Bell J, Valaitis R, Ray S, Keller H. The Sustain and Spread Framework: strategies for sustaining and spreading nutrition care improvements in acute care based on thematic analysis from the More-2-Eat study. BMC Health Serv Res 2018;18:930. doi: 10.1186/s12913-018-3748-8.
The Author(s) 2023