M.J. Cook1,2, M. Lunt3, D.M. Ashcroft4,5, T. Board6, T.W. O’Neill3,7
1. Centre for Health Informatics, Division of Informatics, Imaging and Data Science, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK; 2. National Institute for Health and Care Research Applied Research Collaboration Greater Manchester, The University of Manchester, Manchester, UK; 3. Centre for Epidemiology Versus Arthritis, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology Medicine and Health, University of Manchester, Manchester, UK; 4. Centre for Pharmacoepidemiology and Drug Safety, Division of Pharmacy and Optometry, School of Health Sciences, Manchester Academic Health Science Centre, University of Manchester, UK; 5. NIHR Greater Manchester Patient Safety Translational Research Centre, Faculty of Biology, Medicine and Health, University of Manchester, UK; 6. Department of Trauma and Orthopaedic Surgery, Wrightington Hospital, Wigan, UK; 7. NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, UK
Corresponding Author: Professor Terence O’Neill, Centre for Epidemiology Versus Arthritis, The Stopford Building, University of Manchester, Oxford Road, Manchester, United Kingdom, M13 9PT, Telephone (+44) 0161 3060547, Email terence.o’neill@manchester.ac.uk
J Frailty Aging 2023;12(4)298-304
Published online August 27, 2023, http://dx.doi.org/10.14283/jfa.2023.36
Abstract
BACKGROUND: Among people with hip and knee osteoarthritis (OA), increasing deprivation is associated with reduced likelihood of receiving hip and knee arthroplasty (THA, TKA).
OBJECTIVES: To assess whether higher levels of frailty in the most deprived neighbourhoods explains the association between greater neighbourhood deprivation and reduced likelihood of receiving THA and TKA among people with hip and knee OA.
DESIGN: Longitudinal cohort study.
SETTING: Linked primary and secondary care electronic medical records and national mortality data.
PARTICIPANTS: 104,913 individuals with incident hip OA and 216,420 with incident knee OA.
MEASUREMENTS: Frailty was assessed using a frailty index and categorised as fit, mild, moderate, and severe frailty. Neighbourhood deprivation was assessed using the index of multiple deprivation (IMD).
RESULTS: Compared to those in neighbourhoods in the least deprived quintile of IMD, those in neighbourhoods in the fourth and fifth quintile of IMD (most deprived), respectively, were less likely to receive THA, adjusted subhazard ratio (95% CI), 0.90 (0.87, 0.93) and 0.77 (0.74, 0.80), over a mean follow up of 4.4 years, with similar results for TKA. Higher levels of frailty at OA diagnosis were associated also with reduced likelihood of receiving THA and TKA. The association, however, between deprivation and likelihood of receiving THA and TKA could not be explained by increased levels of frailty among those living in the most deprived areas.
CONCLUSIONS: Further work is needed to understand why those in the most deprived areas are less likely to receive THA and TKA.
Key words: Osteoarthritis, arthroplasty, frailty, deprivation, health inequalities.
Background
Osteoarthritis (OA) is a leading cause of disability among older adults (1). Osteoarthritis affects around 7% of the global population and is responsible for 2% of the total years lived with disability worldwide (1). Osteoarthritis is also associated with significant economic costs, equating to between 0.25% and 0.50% of a country’s gross domestic product according to a systematic review (2). For people with advanced hip and knee OA which is not adequately managed by more conservative therapies, total hip and knee arthroplasty (THA, TKA) are effective interventions. Both THA and TKA are associated with significant improvements in both pain and function (3-5).
There is evidence of inequity in the provision of THA and TKA, with a number of factors, including deprivation, influencing provision. Data from England, Sweden and France indicate that higher levels of deprivation are associated with reduced likelihood of receiving THA and TKA (6-11). One study from England reported that individuals with a higher level of deprivation (defined as being in receipt of means tested benefits) were more likely to need a hip replacement (need assessed using an adapted version of the index of severity of osteoarthritis of the hip) compared to those not receiving benefits (6). However, individuals in need of hip replacement surgery and in receipt of means tested benefits were less likely to be on a waiting list for surgery compared to those in need of hip replacement and not in receipt of benefits (6). Similarly, data from the English Longitudinal Study of Ageing showed that provision of THA and TKA relative to need (need was assessed using a simplified New Zealand score) was lower among those in the most deprived areas (deprivation assessed using the index of multiple deprivation (IMD), compared to those in the least deprived areas (9).
The factors driving the association between deprivation and reduced likelihood of receiving THA and TKA are not well understood. Inequity in access to healthcare services may be relevant, including variation in the provision and quality of services (12). We hypothesised that frailty may also play a role. Individuals with moderate or severe frailty are less likely to have joint surgery. This may be due, in part to a perceived increased risk of complications following surgery among individuals with moderate or severe frailty. One previous study from the UK has indicated that increasing frailty is associated with reduced likelihood of receiving THA among people with hip OA (13). Since frailty is more common in people living in areas of greater deprivation (14), it is possible that this may explain the association between greater deprivation and lower likelihood of receiving THA and TKA, though this hypothesis has not previously been assessed.
The aims of this study were, among those with incident hip and knee OA, first to determine the association between frailty and the likelihood of receiving THA and TKA, second to determine the association between neighbourhood deprivation and frailty at time of incident OA, and third, to determine the association between neighbourhood deprivation and the likelihood of receiving THA and TKA and also whether frailty explains the association between deprivation and likelihood of receiving THA and TKA. Our hypothesis was that higher levels of frailty among those living in the most deprived areas would explain the lower provision of THA and TKA among those living in the most deprived areas.
These data are important to better understand the relationship between neighbourhood deprivation and provision of THA TKA, as well as highlighting the potential impact of frailty on the management of OA.
Methods
Data sources
We used a national database of primary care coded electronic medical records from England; the Clinical Practice Research Datalink (CPRD, Gold and Aurum) (15, 16). The CPRD includes routinely collected electronic medical records from consenting primary care practices. The CPRD comprises two databases, Aurum and Gold, each containing data from primary care practices using different patient management systems. The CPRD is a dynamic database with the number of contributing primary care practices varying over time. As of September 2018, CPRD Aurum included data for around 7 million patients, representing around 13% of the population of England. As of July 2013, CPRD Gold included data for 4.4 million patients, representing around 7% of the total UK population (15). Further details of the CPRD are described elsewhere (15, 16).
The CPRD was linked to secondary care coded electronic medical records, the Hospital Episode Statistics (HES) database (17), and also the Office for National Statistics (ONS) national mortality database.
Identification of incident hip and knee OA
We included in our analysis incident cases of hip and knee OA occurring between 2 January 1998 and 31 March 2019, based on diagnostic codes for hip and knee OA recorded in the primary care records (Supplementary Table 1). The incident date of hip or knee OA was defined as the first occurrence of a hip or knee OA diagnostic code recorded in the primary care record. To mitigate the potential for incorrectly classifying prevalent OA cases as incident cases, we excluded individuals who had a first OA diagnostic code recorded within 12 months of registration with the primary care practice. Since the prevalence of frailty is relatively low among people aged less than 60 years, we included only people aged 60 years or older at the time of OA diagnosis.
Identification of primary total hip and knee arthroplasty
We identified primary THAs and TKAs occurring between 2 January 1997 and 31 March 2019, based on OPCS codes (18) recorded in the HES data. We excluded people who had had a THA or TKA due to fracture, osteonecrosis, rheumatoid arthritis, and malignant neoplasm of bone. In addition, we excluded cases also where the coded primary indication for THA and TKA was used in <0.05% of cases.
Assessment of frailty
Frailty was assessed using the electronic frailty index (eFI) (19). The eFI comprises 36 age-related deficits identified by coded data in primary care electronic medical records and was developed using a standard procedure described by Rockwood, et al (20). Clinical codes were searched in the primary care electronic medical records to identify candidate deficits for inclusion in the eFI recorded based on the deficits included in the Canadian Study of Health and Aging frailty index (21). Further details of the development and initial validation of the eFI are described elsewhere (19). In order to apply the eFI in practices using the SNOMED coding system, we mapped the original eFI Read code lists to SNOMED codes using mapping tables from the National Health Service Data Migration Programme (22). The eFI is calculated as the total number of deficits present in an individual, divided by 36. The deficits included in the eFI is shown in Supplementary Table 2. The eFI was determined at the date of hip or knee OA diagnosis and, based on previously published thresholds, was categorised as fit (eFI≤0.12), mild frailty (0.12<eFI≤0.24), moderate frailty (0.24<eFI≤0.36), and severe frailty (eFI>0.36) (19).The eFI has been validated in multiple databases and criterion validity has been demonstrated by comparing the eFI to other frailty instruments, including the phenotype model of frailty and the Clinical Frailty Scale (19, 23, 24).
Assessment of deprivation
Deprivation was assessed using the 2019 English Index of Multiple Deprivation (IMD) (25). IMD is a multi-dimensional measure of neighbourhood-level deprivation based on an individuals’ postcode and in this analysis IMD was categorised based on quintiles. The IMD is calculated based on seven distinct domains of deprivation: income deprivation, employment deprivation, education, skills, and training deprivation, health deprivation and disability, crime, barriers to housing and services, and living environment deprivation (25). Further details about the construction of the IMD are available elsewhere (26). The 2019 English IMD was categorised into quintiles, based on the national distribution of the IMD.
Assessment of body mass index
Since body mass index (BMI) has been linked with the likelihood of receiving THA and TKA (9), we considered BMI as a covariate in our analyses. Data on body mass index (BMI) was extracted from the primary care record. For each individual, we used the BMI value that was recorded closest to the date of incident OA and within 12 months prior to the date of incident OA. For individuals who did not have BMI recorded in the 12 months prior to the date of incident OA, BMI was set to missing.
Assessment of ethnicity
Since ethnicity has been linked with the likelihood of receiving THA and TKA (27), we considered ethnicity as a covariate in our analyses. Ethnicity was derived from data recorded either in the primary care record, or in the HES data and categorised as White, Asian, Black, Mixed, other. Where there was disagreement in ethnicity category between the primary care records and HES, or where ethnicity was not recorded, ethnicity category was set to missing.
Statistical analysis
Descriptive cohort characteristics were calculated and mean (standard deviation (SD)) values were presented for continuous variables and number (%) were presented for categorical variables.
We determined the association between frailty category at the date of OA diagnosis (in all analyses, ‘fit’ was the reference category) and likelihood of receiving THA or TKA using time-to-event, maximum likelihood, competing-risk regression models (Fine and Grey method) (28), with mortality modelled as a competing event. Individuals contributed person-time to the analysis from the first recorded date of hip or knee OA diagnosis (incident OA) until the date of receiving THA or TKA, date of death, the date the individual’s primary care practice stopped contributing data to the CPRD, or 31 March 2019 (end of study period), whichever came first. We adjusted for year of OA diagnosis, age at OA diagnosis, and sex (based on primary care records). We then adjusted additionally for quintile of IMD in a separate model. In separate analyses, we adjusted additionally for BMI (continuous variable) and ethnicity category among individuals who had these variables recorded.
As a supplementary analysis, we modelled the eFI as a continuous variable when looking at the association between the eFI and likelihood of receiving THA or TKA. To allow for possible nonlinear relationships, we included fractional polynomials for the eFI in a Cox regression model (see Supplementary Text).
We determined the association between quintile of IMD (least deprived was the referent group) and frailty category at the time of hip or knee OA diagnosis using multinomial logistic regression. Quintile of IMD was the exposure variable (least deprived quintile was the referent group) and frailty category was the outcome variable. We adjusted the model for year of OA diagnosis, age at OA diagnosis, and sex. Results were presented as relative risk ratios and 95% CIs.
Finally, we determined the association between quintile of IMD (least deprived was the referent group) and likelihood of receiving THA and TKA using competing risk regression models, with mortality modelled as a competing event. We adjusted for year of OA diagnosis, age at OA diagnosis and sex. We then additionally adjusted for frailty category in a separate model. In separate analyses, we additionally adjusted form BMI (continuous variable) and ethnicity category among individuals who had these variables recorded. The results were expressed as subhazard ratios (SHR) and 95% confidence intervals (CI).
Analyses were carried out using Stata/MP v13.1.
The protocol for this work was approved by the Independent Scientific Advisory Committee for CPRD research (protocol number 20_119). CPRD has ethics approval from the Health Research Authority to support research using anonymised patient data.
Results
Study participants
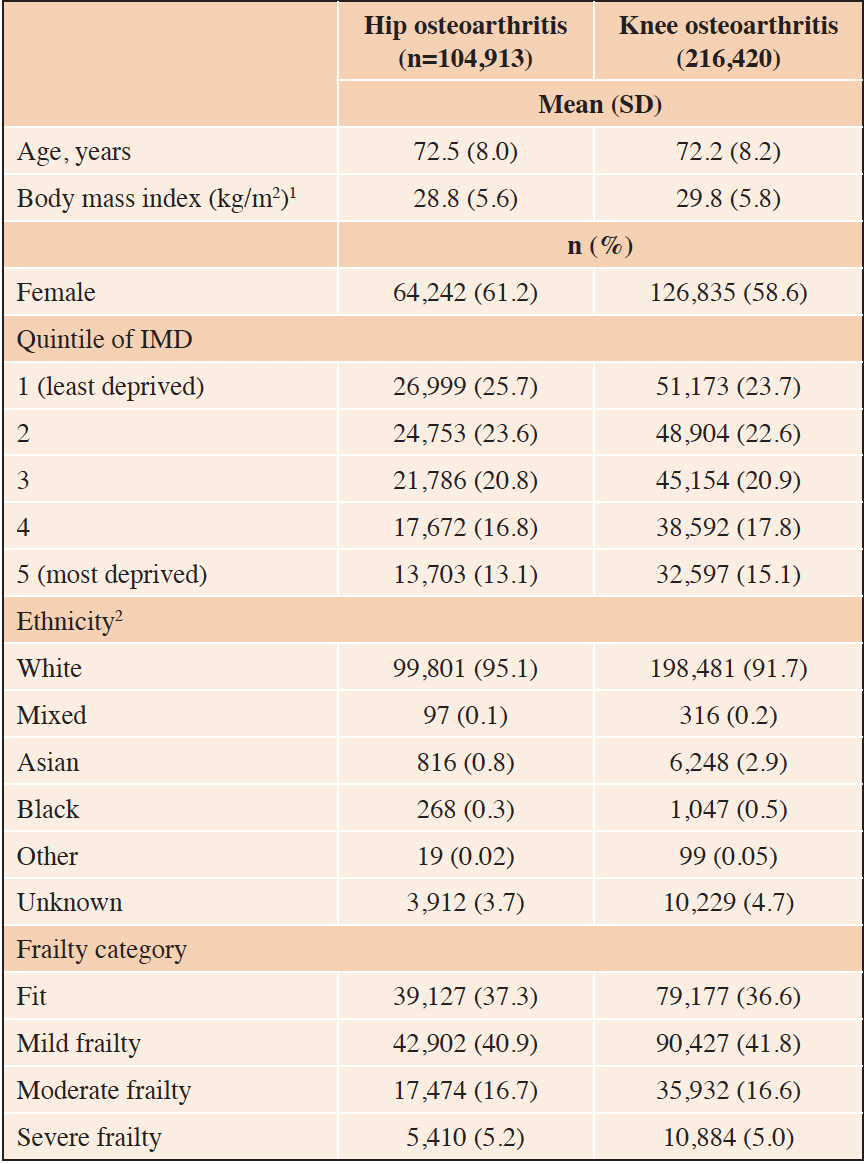
We identified 105,029 people with incident hip OA and 216,708 with incident knee OA. After excluding 116 and 288 people with hip and knee OA, respectively where the first OA diagnostic code was recorded within 1 year of registration with the primary care practice, 104,913 people with hip OA and 216,420 with knee OA contributed to the analysis (Supplementary Figure 1). Among those with hip and knee OA, respectively, the mean (SD) age at the date of incident OA was 72.5 (8.0) and 72.2 (8.2) years and 61.2% and 58.6% were female. At the date of incident hip OA and knee OA, respectively, the proportion of individuals who were; fit was 37.3% and 36.6%; had mild frailty was 40.9% and 41.8%; moderate frailty was 16.7% and 16.6%; and severe frailty was 5.2% and 5.0% (Table 1). The number and proportion of participants in the hip OA and knee OA cohorts in each quintile of IMD is shown in Table 1.
1. Body mass index was recorded for 41,693 individuals with hip osteoarthritis (40% of the hip cohort) and 87,872 individuals with knee osteoarthritis (41% of the knee cohort); 2. Ethnicity was recorded for 101,001 people in the hip cohort (96% of the hip cohort) and 206,191 people in the knee cohort (95% of the knee cohort)
BMI was recorded in the year prior to the date of incident OA for 41,693 people in the hip OA cohort (40% of the hip OA cohort) and 87,872 people in the knee OA cohort (41% of the knee OA cohort) (Table 1). Ethnicity was recorded for 101,001 individuals in the hip cohort (96% of the hip cohort) and 206,191 individuals in the knee cohort (95% of the knee cohort). There were no missing data for frailty, age, sex, or IMD.
The mean (SD) follow up among those with hip and knee OA, respectively was 4.4 (4.5) and 5.4 (4.6) years. During follow up, 34,099 (32.5%) of individuals with hip OA had a THA and 36,067 (16.7%) of individuals with knee OA had a TKA. The crude number and percentage of individuals in the hip OA and knee OA cohorts, respectively who received a THA and a TKA, stratified by frailty is shown in Supplementary Table 3, and stratified by quintile of IMD is shown in Supplementary Table 4.
Association between frailty at time of OA diagnosis and likelihood of receiving THA and TKA
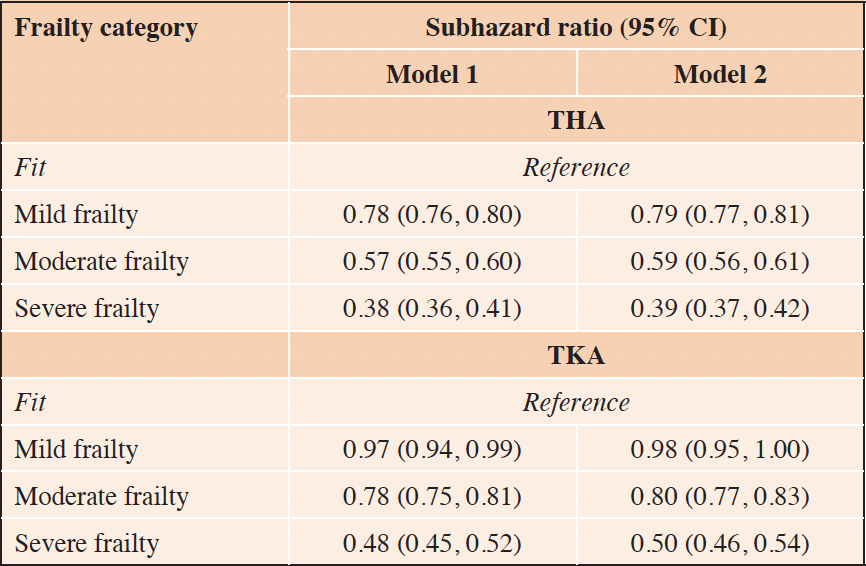
Increasing frailty at the time of OA diagnosis was associated with reduced likelihood of receiving both THA and TKA (Table 2). Adjusting for quintile of IMD had little impact on the association between frailty and likelihood of receiving THA and TKA (Table 2). In a model adjusted for year of OA diagnosis, age at OA diagnosis, sex and quintile of IMD, compared to those who were ‘fit’, the subhazard ratio (95%C) for receiving THA among those with mild, moderate, and severe frailty, respectively, was 0.79 (0.77, 0.81), 0.59 (0.56, 0.61), and 0.39 (0.37, 0.42). The corresponding values for receiving TKA were 0.98 (0.95, 1.00), 0.80 (0.77, 0.83), and 0.50 (0.46, 0.54) (Table 2). We saw similar results in the subset of individuals who had BMI and ethnicity recorded and adjusting additionally for BMI and ethnicity had little impact on the association between frailty and likelihood of receiving THA and TKA (Supplementary tables 5 and 6).
In a supplementary analysis, we analysed the eFI as a continuous variable (see Supplementary Text and Supplementary Figure 2). There was evidence of a nonlinear relationship between the eFI and receiving a THA and TKA (Supplementary Figure 2), however this did not change the interpretation of the results presented in our primary analysis.
Model 1: adjusted for year of OA diagnosis, age at OA diagnosis and sex; Model 2: adjusted for year of OA diagnosis, age at OA diagnosis, sex, and quintile of IMD
Association between deprivation and frailty at the date of incident OA
Increasing quintile of IMD (increasing neighbourhood deprivation) was associated with increasing risk of mild, moderate, and severe frailty (Table 3). In the hip OA cohort, compared to fit individuals in the least deprived quintile of IMD, the relative risk ratio (95% CI) of severe frailty at the time of OA diagnosis among those in the most deprived quintile of IMD was 4.16 (3.77, 4.59), in a model adjusted for year of OA diagnosis, age, and sex. The corresponding result in the knee OA cohort was 3.47 (3.23, 3.72) (Table 3).
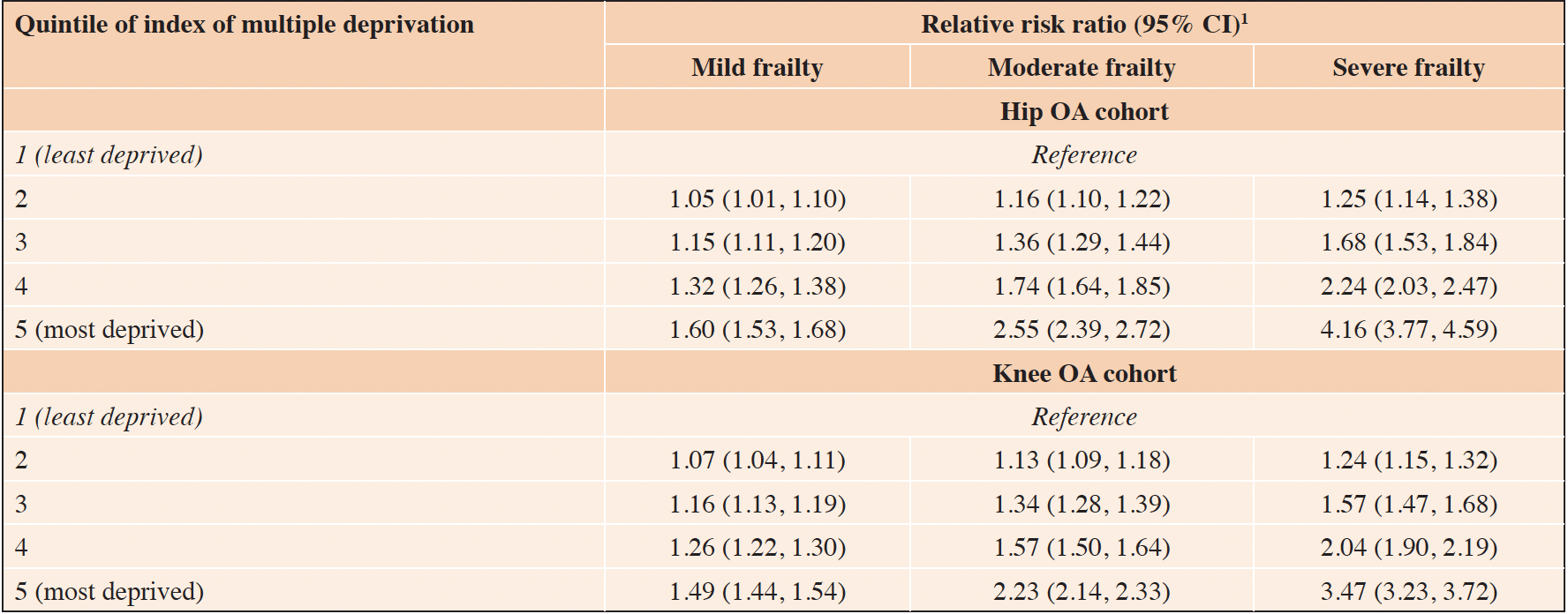
Table 3. Relative risk ratio for frailty category at date of OA diagnosis by quintile of index of multiple deprivation
1. Multivariable logistic regression model adjusted for year of diagnosis of OA, age, and sex. ‘Fit’ was the reference category.
Association between neighbourhood deprivation and likelihood of receiving THA and TKA
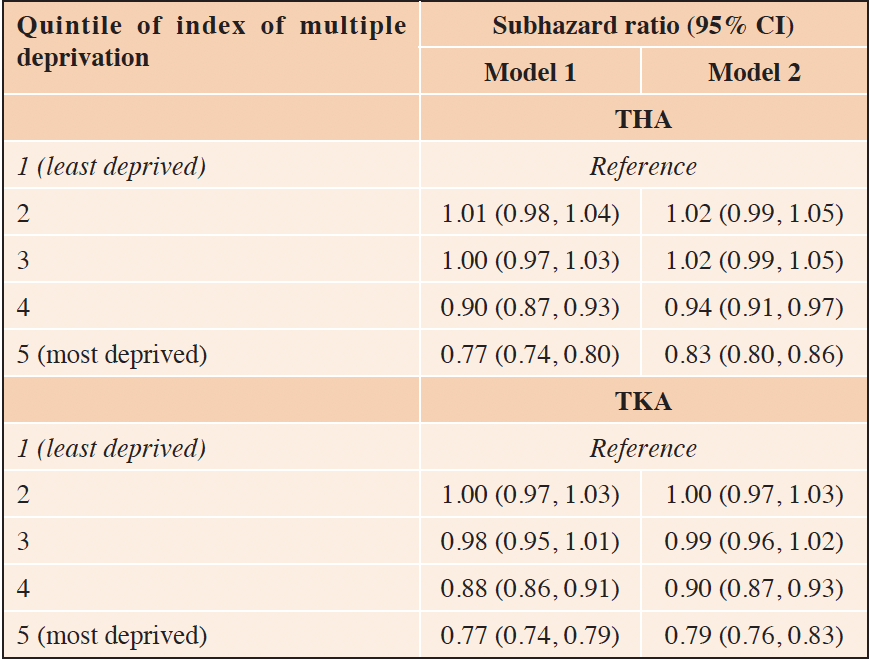
Compared to those in neighbourhoods in the least deprived quintile of IMD, those in neighbourhoods in the 4th and 5th (most deprived) quintiles of IMD were less likely to receive THA and TKA (Table 4). In a model adjusted for year of OA diagnosis, age at OA diagnosis, sex and frailty category at time of OA diagnosis, compared to those in neighbourhoods in the least deprived quintile of IMD, the subhazard ratio (95% CI) for receiving THA among those in neighbourhoods in the 2nd, 3rd, 4th, and 5th quintile of IMD, respectively was 1.02 (0.99, 1.05), 1.02 (0.99, 1.05), 0.94 (0.91, 0.97), and 0.83 (0.80, 0.86). The corresponding values for TKA were 1.00 (0.97, 1.03), 0.99 (0.96, 1.02), 0.90 (0.87, 0.93), and 0.79 (0.76, 0.83) (Table 4). There was no important difference in the strength of these associations after adjustment for frailty category (Table 4).
Model 1: adjusted for year of OA diagnosis, age at OA diagnosis and sex; Model 2: adjusted for year of OA diagnosis, age at OA diagnosis, sex, and eFI category at time of OA diagnosis
We saw similar results in the subset of individuals who had BMI and ethnicity recorded and adjusting additionally for BMI and ethnicity had little impact on the association between IMD and likelihood of receiving THA and TKA (Supplementary tables 7 and 8).
Discussion
We found that both increasing frailty at the time of OA diagnosis and increasing deprivation were associated with reduced likelihood of receiving THA and TKA, independently of each other. We also found a ‘dose-response’ association between increasing neighbourhood deprivation and increased frailty at the time of OA diagnosis. Adjusting for frailty at the time of OA diagnosis attenuated the association between neighbourhood deprivation and reduced likelihood of receiving THA, though did not fully explain the association. Frailty had little impact of the association between neighbourhood deprivation and likelihood of receiving TKA.
As outlined in the introduction, an association between increasing deprivation and reduced likelihood of receiving THA and TKA, both in the general population and also among people with hip and knee OA, has previously been reported (6-11), although factors explaining this association are not well understood. One previous study using data from the English Longitudinal Study of Ageing looked at variation in provision of THA and TKA by deprivation relative to need (need was assessed using a simplified New Zealand score) (9). In agreement with our findings, the authors found lower rates of THA and TKA among those in the most deprived (compared to least deprived) quintile of IMD); rate ratio (95% CI) for THA and TKA, respectively 0.31 (0.30, 0.33) and 0.33 (0.31, 0.34). The magnitude of the association was more marked than observed in our study, however, we did not have information about provision relative to need. Previous data have suggested that need is greater among those most deprived, compared to those least deprived (9). One previous study from the UK looked at the association between frailty, assessed using the eFI, and likelihood of receiving THA among people with hip OA (13). The study found, similar to our findings, that increasing frailty was associated with reduced likelihood of receiving THA (13). In a model adjusted for age, sex, region and year of OA diagnosis, compared to individuals with ≤4 eFI deficits, the hazard ratio (95% CI) for receiving THA among those with 5-8 deficits, 9-12 deficits, and ≥13 deficits respectively, was 0.77 (0.74, 0.81), 0.52 (0.47, 0.59), 0.34 (0.22, 0.51) (13). These results match closely with our findings. Slight differences in the hazard ratios may due to differences in the study cohort and study period.
Our study has a number of strengths, including a large sample size, linked primary and secondary care data, and the use of a well validated frailty index. There are also limitations which need to be considered when interpreting our findings. We used the IMD as a measure of neighbourhood deprivation based on an individual’s postcode. Caution is needed when interpreting the association between neighbourhood deprivation and likelihood of receiving THA and TKA, since neighbourhood deprivation is not equivalent to individual-level deprivation. We assessed frailty at a fixed point in time (the date of hip or knee OA diagnosis). We did not assess the impact of change in frailty status during follow up on the likelihood of receiving THA and TKA. However, due to the relatively short follow up period in our study, it is unlikely that there was significant change in frailty during follow up. In our analysis, we were not able to account for OA disease severity which may impact on the need for THA and TKA. It seems unlikely though that those who live in the most deprived areas or are frail would tend to have less severe OA and therefore less need for THA and TKA. Indeed, one study of individuals who received total joint arthroplasty found higher OA disease severity (Kellgren-Lawrence grade) among those with a low, compared to higher income (29). Whilst it is plausible that more severely frail patient may be less willing to undergo THA and TKA (and also healthcare professionals less willing to carry out THA and TKA), there is no evidence of reduced willingness to undergo THA or TKA among those living in more deprived compared to less deprived areas (30). Indeed one study looking at the association between education and willingness to undergo THA and TKA following first-line intervention for OA found lower willingness to undergo THA and TKA among those with higher educational attainment (31).
We identified hip and knee OA based on diagnostic codes in primary care electronic medical records. In a validation study including a random sample of 170 individuals in the CPRD age 65 years and over with a hip OA code were identified and their primary care physicians were contacted and asked to review and confirm the diagnosis of hip OA based on medical records and x-rays (32). Of those with a hip OA code, 80% of individuals had a confirmed diagnosis using x-ray and 88% of individuals had a confirmed diagnosis (either by clinical means or by x-ray) suggesting that codes for hip OA in the CPRD dataset are likely to accurately identify true cases (32).
Being overweight or obese has been linked with increased incidence of THA and TKA among people with hip and knee OA (33). In our data, BMI was only available for about 40% of the study cohort. However, among individuals who had BMI recorded additional adjustment for BMI had only a marginal impact on the association between both deprivation and frailty and also deprivation and the likelihood of receiving THA and TKA.
Previous studies have indicated a reduced likelihood of receiving THA and TKA among individuals belonging to an ethnic minority group (27). Additional adjustment for ethnicity, however, had little impact on the association between deprivation and likelihood of receiving THA and TKA, or on the association between frailty and likelihood of receiving THA and TKA in our analyses.
Factors driving the association between neighbourhood deprivation and likelihood of receiving THA and TKA are not fully understood. In the early 1970s, Hart described how “the availability of good medical care tends to vary inversely with the need of the population served” (34), with inequities in the provision and use of services in the UK also observed more recently (35). Variation in the likelihood of receiving THA and TKA between areas of differing deprivation levels do not appear to be explained by differences in primary care consultation rates for OA. Indeed, one study using data from the CPRD reported that primary care consultation rates for OA were higher in the most deprived compared to leased deprived areas (36). However, despite this, people in more deprived areas may be less likely to be on a waiting list for THA and TKA (6). Further work is needed to determine factors that influence why people living in more deprived areas are less likely to have joint replacement surgery, including access to primary and secondary healthcare, pathways of care, and also patient expectations and views of arthroplasty.
In conclusion, our analysis suggested that both increased deprivation and increased frailty at the time of incident hip or knee OA were associated with reduced likelihood of receiving THA and TKA. Frailty partially, though did not fully explain the reduced likelihood of receiving THA among those most deprived. Further work is needed to better understand the factors driving the association between higher deprivation and reduced likelihood of receiving THA and TKA among people with OA.
Acknowledgements: This study is based on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the National Health Service (NHS) as part of their care and support. The Office for National Statistics is the provider of the ONS data contained within the CPRD data. Hospital Episode Data and the ONS data, © 2022, are reused with the permission of NHS Digital. All rights reserved. The protocol for this work was approved by the Independent Scientific Advisory Committee for CPRD research (protocol number 20_119). The authors acknowledge the assistance given by IT Services and the use of the Computational Shared Facility at The University of Manchester.
Funding: Michael J Cook was funded by a National Institute for Health Research Doctoral Research Fellowship for this research project. This work was supported by Versus Arthritis (grant number 21755) and the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007). Darren M Ashcroft is funded by the NIHR Greater Manchester Patient Safety Translational Research Centre (PSTRC-2016-003). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care or Public Health England.
Competing interests: All authors declare no competing interests.
Ethics approval: The protocol for this work was approved by the Independent Scientific Advisory Committee for CPRD research (protocol number 20_119). CPRD has ethics approval from the Health Research Authority to support research using anonymised patient data.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745-59; DOI: https://doi.org/10.1016/S0140-6736(19)30417-9.
2. Puig-Junoy J, Ruiz Zamora A. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Seminars in arthritis and rheumatism. 2015;44(5):531-41; https://doi.org/10.1016/j.semarthrit.2014.10.012.
3. Sabah SA, Knight R, Alvand A, Beard DJ, Price AJ. Early patient-reported outcomes from primary hip and knee arthroplasty have improved over the past seven years. The bone & joint journal. 2022;104-B(6):687-95; DOI: https://doi.org/10.1302/0301-620X.104B6.BJJ-2021-1577.R1.
4. Beard DJ, Harris K, Dawson J, Doll H, Murray DW, Carr AJ, et al. Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. Journal of clinical epidemiology. 2015;68(1):73-9; DOI: https://doi.org/10.1016/j.jclinepi.2014.08.009.
5. Judge A, Arden NK, Kiran A, Price A, Javaid MK, Beard D, et al. Interpretation of patient-reported outcomes for hip and knee replacement surgery: identification of thresholds associated with satisfaction with surgery. The Journal of bone and joint surgery British volume. 2012;94(3):412-8; DOI: https://doi.org/10.1302/0301-620X.94B3.27425.
6. Milner PC, Payne JN, Stanfield RC, Lewis PA, Jennison C, Saul C. Inequalities in accessing hip joint replacement for people in need. European Journal of Public Health. 2004;14(1):58-62; DOI: https://doi.org/10.1093/eurpub/14.1.58.
7. Yong PFK, Milner PC, Payne JN, Lewis PA, Jennison C. Inequalities in access to knee joint replacements for people in need. Annals of the rheumatic diseases. 2004;63(11):1483-9; DOI: http://dx.doi.org/10.1136/ard.2003.013938.
8. Dixon T, Shaw M, Ebrahim S, Dieppe P. Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Annals of the rheumatic diseases. 2004;63(7):825-30; DOI: http://dx.doi.org/10.1136/ard.2003.012724.
9. Judge A, Welton NJ, Sandhu J, Ben-Shlomo Y. Equity in access to total joint replacement of the hip and knee in England: cross sectional study. Bmj. 2010;341:c4092; DOI: https://doi.org/10.1136/bmj.c4092.
10. Wetterholm M, Turkiewicz A, Stigmar K, Hubertsson J, Englund M. The rate of joint replacement in osteoarthritis depends on the patient’s socioeconomic status. Acta orthopaedica. 2016;87(3):245-51; DOI: https://doi.org/10.1136/bmj.c4092.
11. Michel M, Bryere J, Maravic M, Marcelli C. Knee replacement incidence and social deprivation: results from a French ecological study. Joint, bone, spine : revue du rhumatisme. 2019;86(5):637-41; DOI: https://doi.org/10.1016/j.jbspin.2019.03.004.
12. Majeed FA, Chaturvedi N, Reading R, Ben-Shlomo Y. Equity in the NHS. Monitoring and promoting equity in primary and secondary care. Bmj. 1994;308(6941):1426-9; DOI: https://doi.org/10.1136/bmj.308.6941.1426.
13. Ferguson R, Prieto-Alhambra D, Peat G, Delmestri A, Jordan KP, Strauss VY, et al. Influence of pre-existing multimorbidity on receiving a hip arthroplasty: cohort study of 28 025 elderly subjects from UK primary care. Bmj Open. 2021;11(9):e046713; DOI: http://dx.doi.org/10.1136/bmjopen-2020-046713.
14. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365-75; DOI: https://doi.org/10.1016/S0140-6736(19)31786-6.
15. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). International journal of epidemiology. 2015;44(3):827-36; DOI: https://doi.org/10.1093/ije/dyv098.
16. Wolf A, Dedman D, Campbell J, Booth H, Lunn D, Chapman J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. International journal of epidemiology. 2019;48(6):1740-g; DOI: https://doi.org/10.1093/ije/dyz034.
17. Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data Resource Profile: Hospital Episode Statistics Admitted Patient Care (HES APC). International journal of epidemiology. 2017;46(4):1093-i; DOI: https://doi.org/10.1093/ije/dyx015.
18. National Joint Registry. OPCS Codes relevant to procedures recorded on the NJR. 2018 [cited 17/08/2021]; Available from: https://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Data%20collection%20forms/OPCS%20Procedure%20codes%20relevant%20to%20NJRv6%2020180413.pdf?ver=2019-03-24-221729-620
19. Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age and ageing. 2016;45(3):353-60; DOI: https://doi.org/10.1093/ageing/afw039.
20. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8; DOI: https://doi.org/10.1186/1471-2318-8-24.
21. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2005;173(5):489-95; DOI: https://doi.org/10.1503/cmaj.050051.
22. NHS Digital. Read Version 2 to SNOMED CT mapping tables. 2020 [cited 17/08/2021]; Available from: https://isd.digital.nhs.uk/trud3/user/authenticated/group/0/pack/9
23. Brundle C, Heaven A, Brown L, Teale E, Young J, West R, et al. Convergent validity of the electronic frailty index. Age and ageing. 2019;48(1):152-6; DOI: https://doi.org/10.1093/ageing/afy162.
24. Hollinghurst J, Fry R, Akbari A, Clegg A, Lyons RA, Watkins A, et al. External validation of the electronic Frailty Index using the population of Wales within the Secure Anonymised Information Linkage Databank. Age and ageing. 2019;48(6):922-6; DOI: https://doi.org/10.1093/ageing/afz110.
25. Ministry of Housing Communities and Local Government. English indices of deprivation 2019: research report. 2019 02/08/2022 [cited; Available from: https://www.gov.uk/government/publications/english-indices-of-deprivation-2019-research-report
26. Ministry of Housing Communities and Local Government. The English Indices of Deprivation 2019 – Technical Report. 2019 08/08/2022 [cited; Available from: https://www.gov.uk/government/publications/english-indices-of-deprivation-2019-technical-report
27. Chun DS, Leonard AK, Enchill Z, Suleiman LI. Racial Disparities in Total Joint Arthroplasty. Curr Rev Musculoskelet Med. 2021; https://doi.org/10.1007/s12178-021-09718-3
28. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496-509; DOI: https://doi.org/10.1080/01621459.1999.10474144.
29. Suleiman LI, Manista GC, Sherman AE, Adhia AH, Karas V, Sporer SM, et al. The Impact of Race and Socioeconomic Status on Total Joint Arthroplasty Care. J Arthroplasty. 2021;36(8):2729-33; DOI: https://doi.org/10.1016/j.arth.2021.03.002.
30. Hawker GA, Wright JG, Glazier RH, Coyte PC, Harvey B, Williams JI, et al. The effect of education and income on need and willingness to undergo total joint arthroplasty. Arthritis & Rheumatism. 2002;46(12):3331-9; DOI: https://doi.org/10.1002/art.10682.
31. Dell’Isola A, Jönsson T, Rolfson O, Cronström A, Englund M, Dahlberg L. Willingness to Undergo Joint Surgery Following a First-Line Intervention for Osteoarthritis: Data From the Better Management of People With Osteoarthritis Register. Arthritis care & research. 2021;73(6):818-27; DOI: https://doi.org/10.1002/acr.24486.
32. Ferguson RJ, Prieto-Alhambra D, Walker C, Yu D, Valderas JM, Judge A, et al. Validation of hip osteoarthritis diagnosis recording in the UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf. 2019;28(2):187-93; DOI: https://doi.org/10.1002/pds.4673.
33. Burn E, Murray DW, Hawker GA, Pinedo-Villanueva R, Prieto-Alhambra D. Lifetime risk of knee and hip replacement following a GP diagnosis of osteoarthritis: a real-world cohort study. Osteoarthritis and cartilage. 2019;27(11):1627-35; DOI: https://doi.org/10.1016/j.joca.2019.06.004.
34. Hart JT. The inverse care law. Lancet. 1971;1(7696):405-12; DOI: doi: 10.1016/s0140-6736(71)92410-x.
35. McLean G, Guthrie B, Mercer SW, Watt GC. General practice funding underpins the persistence of the inverse care law: cross-sectional study in Scotland. Brit J Gen Pract. 2015;65(641):e799-e805; DOI: https://doi.org/10.3399/bjgp15X687829.
36. Yu D, Jordan KP, Wilkie R, Bailey J, Fitzpatrick J, Ali N, et al. Persistent inequalities in consultation incidence and prevalence of low back pain and osteoarthritis in England between 2004 and 2019. Rheumatology Advances in Practice. 2022;7(1); DOI: https://doi.org/10.1093/rap/rkac106.
37. Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. International journal of epidemiology. 1999;28(5):964-74; DOI: https://doi.org/10.1093/ije/28.5.964.
The Author(s) 2023