R.C. Castrejón-Pérez1, S.A. Borges-Yáñez2, R. Ramírez-Aldana1,5,6, I. Nasu3, Y. Saito4
1. Instituto Nacional de Geriatría, National Institutes of Health, Ministry of Health, Mexico; 2. Postgraduate and Research Studies Division, Dentistry School, National Autonomous University of Mexico, Mexico; 3. School of Dentistry, Matsudo Nihon University, Chiba, Japan; 4. College of Economics, Nihon University, Tokyo, Japan; 5. Escuela Superior de Ingeniería y Tecnología, Universidad Internacional de la Rioja, España; 6. Departamento de Matemáticas, Facultad de Ciencias, Universidad Nacional Autónoma de México
Corresponding Author: Roberto Carlos Castrejón-Pérez, PhD, Instituto Nacional de Geriatría, National Institutes of Health, Ministry of Health, Mexico, rcastrejon@inger.gob.mx, rc.castrejon.perez@gmail.com
J Frailty Aging 2024;in press
Published online January 31, 2024, http://dx.doi.org/10.14283/jfa.2024.10
Abstract
BACKGROUND: Oral health is a relevant component for overall health. Oral disease onset at an early age and may harm several health dimensions, especially among older people, and has been associated with frailty.
OBJECTIVE: To evaluate associations between the Frailty Index (FI) and self-reported oral diseases among older, community-dwelling Japanese people.
DESIGN: Cross-sectional and prospective analyses were performed.
SETTING AND PARTICIPANTS: We analyzed data from 2,529 participants at the baseline and four-year follow-up of the Nihon University Japanese Longitudinal Study of Aging, which had a four-year follow-up.
MEASUREMENTS: We used the self-reported number of teeth, self-reported satisfaction with dentures, and self-reported ability to chew hard food as independent variables. We computed an FI that included 40 deficits as the dependent variable. The FI score ranged from 0 to 1, with a higher score associated with adverse health outcomes and mortality. Considering a gamma distribution and controlling for age, gender, marital status, education, working status, and residence area, we fitted generalized linear models.
RESULTS: We found that dissatisfied denture users had a 2.1% (95% CI 1.006–3.279) higher frailty score than non-denture users at the baseline and a 2.1% (95% CI 0.629–3.690) higher frailty score than non-denture users at the four-year follow-up. In the cross-sectional analysis, with each additional reported tooth at the baseline, the FI score was lower by 1.5% (95% CI -2.878 to -0.208) at the four-year follow-up. In both the cross-sectional and the prospective analyses, the FI scores increased as the ability to chew hard food decreased.
CONCLUSIONS: Self-reported oral diseases are associated with the FI score cross-sectionally and prospectively. Identifying factors prospectively associated with frailty may improve strategies for the next generation of older people. Considering oral diseases may help clinicians personalize treatment plans for older people.
Key words: Frailty index, number of teeth, ability to chew, satisfaction with dentures, cohort study.
Introduction
The World Health Organization recognized oral health as a relevant component of overall health in the Global Oral Health Status Report 2022 (1). The onset of oral diseases occurs early in life, and their impact accumulates over one’s lifetime, becoming more severe in middle and older age (1, 2). Oral diseases share some risk factors with noncommunicable diseases (1-5) and impact additional domains of health and living (1, 5–9). Available evidence indicates that oral diseases are associated with changes in food selection and poor nutritional status (9–11) and with poor quality of life (12, 13). Other studies have reported that oral diseases are associated with higher mortality rates among older people (7, 8).
Frailty, recognized as a public health challenge (14, 15), is a state of increased vulnerability to outer and inner stressors due to a diminished physiological response. Frail individuals are prone to adverse health outcomes (14, 16, 17). The number of studies reporting an association between oral diseases and frailty has increased in recent years (18–27). The results of these studies support a functional path linking oral diseases and frailty (28), since the most common oral diseases associated with frailty may also harm oral function (e.g., number of natural teeth, periodontal status, occlusal force, reduced ability to chew hard food, tongue pressure, diadochokinesis, swallowing difficulties, reporting dry mouth, prosthetic characteristics, and dental decay) (18, 25, 26, 29, 30), therefore compromising nutritional status from earlier ages and for an extended period of life.
Although the association between oral diseases and frailty has been reported in different populations (20, 21, 24, 26, 31–33), most of these studies included objectively measured oral diseases, making data collection challenging and expensive due to the clinical training and standardized procedures required for accurate and valid data collection. Moreover, most prospective studies included regionally representative participants or relatively short follow-up periods (31, 33–35). This study aimed to evaluate the association between three self-reported oral diseases and the Frailty Index (FI) at baseline and at the four-year follow-up among older Japanese people.
Methods
This study included cross-sectional and prospective analyses of nationally representative older people participating in the Nihon University Japanese Longitudinal Study of Aging (36, 37). The sample was selected using a multistage stratified sampling method. The participants were selected from the National Registry List, the eligible voter list, and the list of housing units developed by Central Research Services Incorporated. Potential participants were 65 and over; those 75 years and over were oversampled (38). The participants were interviewed at the baseline (in 1999), in the second wave in 2001, and in the third wave in 2003. We included 2,529 participants who were interviewed at the baseline and in the third wave and had no missing values in any variable considered for the analyses.
Variables
The self-reported number of natural teeth (number of teeth; range 0–32) was measured by asking “How many original teeth do you have?” Utilization of and satisfaction with dentures were assessed by asking, “Do you have dentures? (no/yes).” Those who responded “no” were considered “non-denture users.” Those who answered “yes” were asked, “Are you satisfied with your dentures?” (no/yes). Those who responded “yes” were considered “satisfied denture users.” Those who answered “no” were considered “dissatisfied denture users.” The ability to chew hard food was measured using the question “What is the hardest group of food you are able to bite and chew?” The offered responses included an ordered list of six groups of food ranging from the most challenging (hardest) to the softest (Group 1: hard dried squid or pickled radish; Group 2: boiled pork meat or raw carrots or celery; Group 3: deep-fried tofu, pickled octopus, pickled Chinese cabbage, or raisins; Group 4: rice, apples, fish cake, or boiled asparagus; Group 5: bananas, boiled beans, canned corned beef, or wafers; Group 6unable to chew the food listed in Group 5).
The FI was the dependent variable, computed using 40 components, including symptoms, signs, disabilities, and diseases, as suggested by Searle and colleagues (39). To calculate the FI, each item was categorized as 0 if the deficit was absent and 1 if the deficit was present. The variables with ordinal scales of three options were coded with 0 if the deficit was absent, 0.5 if the deficit was partially present, and 1 if the deficit was present. Self-rated health with an ordinal response of five options was coded as 0, 0.25, 0.5, 0.75, and 1 (Supplementary Table A). The values of all responses were summed and divided by 40 to obtain a FI score ranging from 0 to 1 with a gamma distribution (39), with a higher score associated with adverse health outcomes and mortality (40).
Age (65 years old and over), sex (male or female), marital status (married, single or divorced, or widowed), educational background (completed Grade 9, completed Grade 12, or completed junior college or higher), current working status (working, retired and unemployed, retired and engaged with housework, or retired and no longer doing housework), and residence area (urban or rural) were included as covariates.
Statistical analysis
We present descriptive analyses with percentages, mean values, and standard deviation, and minimum, maximum, and median values when appropriate. We fitted generalized linear models using a gamma distribution and identity link function for both the cross-sectional and prospective approaches, with each self-reported oral condition and covariate serving as an explanatory variable (univariate models). In the multivariable analyses, we fitted similar generalized linear models. We included the control variables from the univariate analyses and each separate self-reported oral condition as explanatory variables. The reason for fitting a multivariable model for each self-reported oral condition is that there is collinearity between them. Thus, we fitted four cross-sectional and four prospective multivariable models. The FI score was multiplied by 100 so that it could be interpreted as a percentage of change by the unit of change in the independent variables of interest. All analyses used Stata version 15 (StataCorp LLC, College Station, TX, USA).
Results
Of the 2,529 participants included in the analyses, 56.8% were women. The women were, on average, older than the men (74.8 SD 5.9 vs 73.7 SD 6.0, respectively). Almost two-thirds of the participants were married, 33.7% were widowed, and 58.3% lived in urban areas. Most women had completed Grade 9, while 14.3% of the men had completed junior college or higher. The majority (60.4%) of the men were retired and unemployed, while half of the women were retired and engaged in housework. Regarding self-reported oral diseases, the median number of teeth was higher among the men than among the women (9 and 5, respectively), a higher proportion of the men was not using dentures, and almost two-thirds of the women were satisfied denture users. Concerning the ability to chew, most participants reported being able to chew the most challenging food group, whereas 7.1% reported being able to bite and chew only the three softest food groups (Table 1).
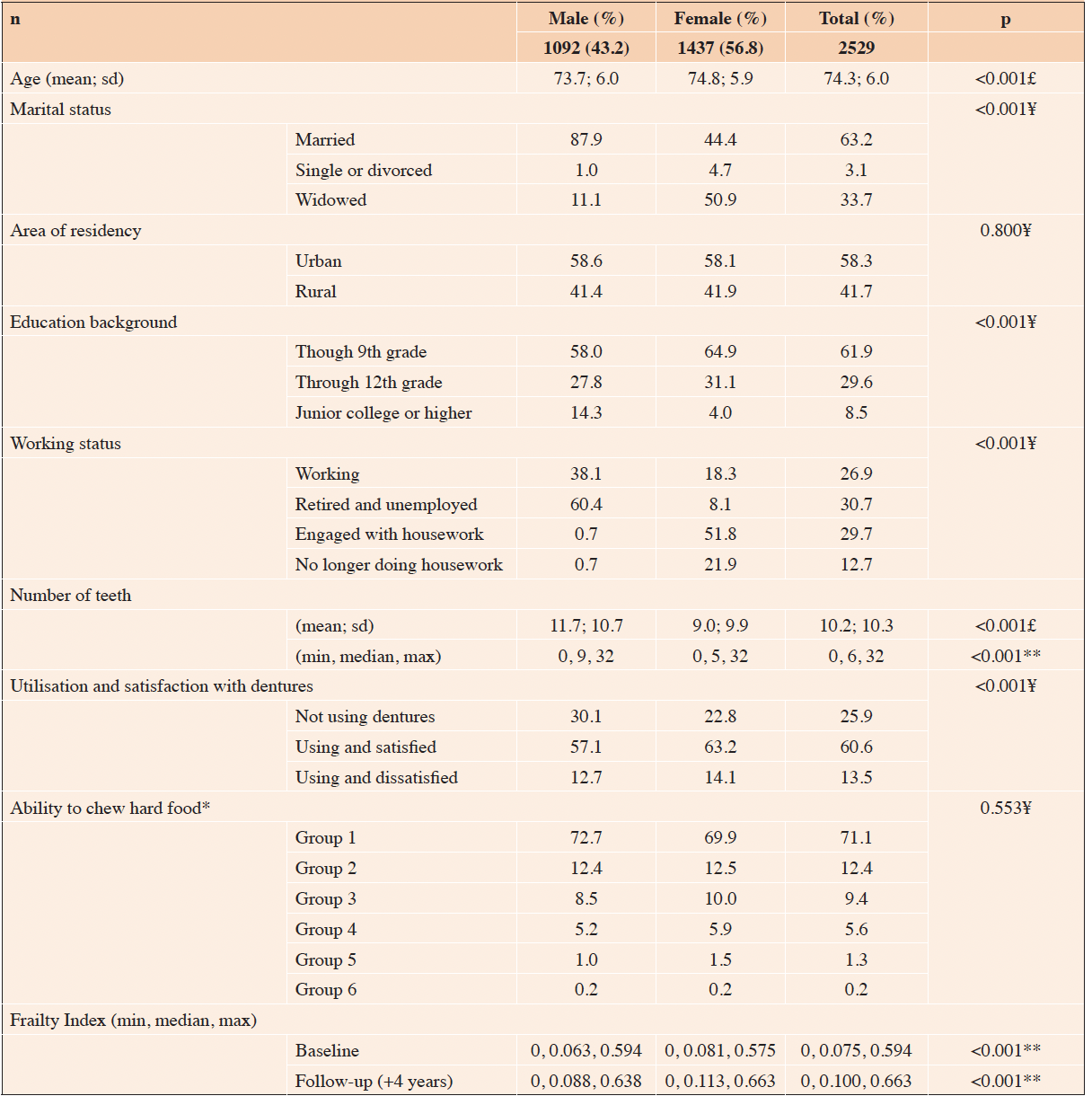
Table 1. Demographics, self-reported oral diseases, and the Frailty Index among community-dwelling older Japa-nese people
* Group 1-Hard dried squid or pickled radish; Group 2-Boiled pork meat, raw carrots, or celery; Group 3-Deep-fried tofu, pickled octopus, tsukemono made from Chinese cabbage, or raisins; Group 4-Rice, apples, fish cake, or boiled asparagus; Group 5-Bananas, boiled beans, canned corned beef, or wafers; Group 6-Unable to chew food listed in Group 5; FI: Frailty Index; £ t-test; ¥ chi2; ** Kruskal-Wallis
Cross-sectional approach
The univariate analyses showed that age, being female, single or widowed, retired and unemployed, retired and engaged with housework, retired and no longer doing housework, a dissatisfied denture user, and able to chew only softer foods were associated with a higher FI score at the baseline. Conversely, having completed Grade 12, junior college, or higher, and each additional tooth reported were associated with a lower FI score at the baseline. The covariates that remained associated with higher FI scores in the multivariable analyses were age and working status (Supplementary Table B). Regarding self-reported oral diseases, being a dissatisfied denture user and able to chew food from Groups 2–6 were associated with a higher FI score at the baseline (Table 2).
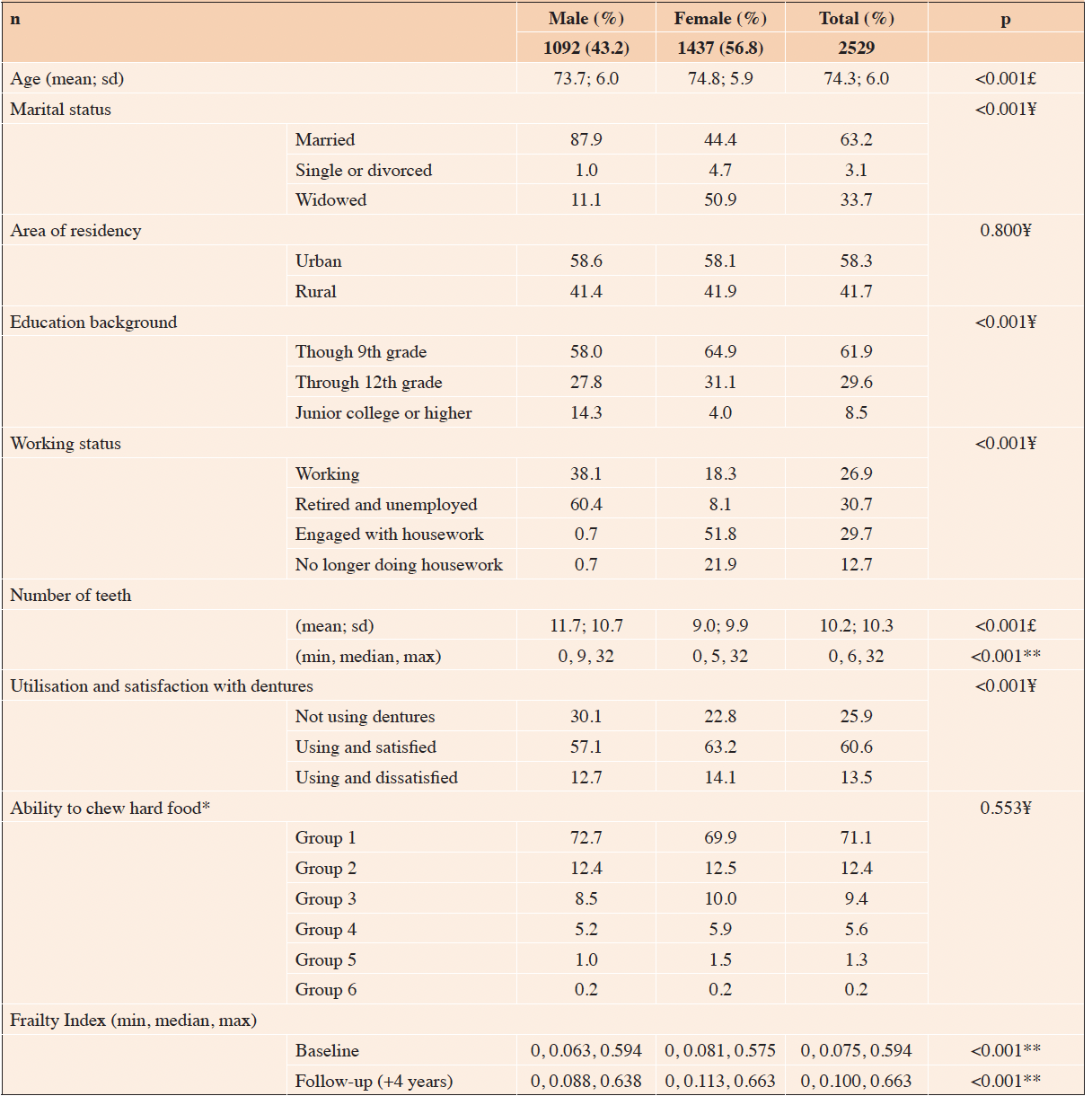
Table 2. Univariate generalized linear regression models between covariates, self-reported oral diseases, and the Frailty Index among community-dwelling older Japanese people: Cross-sectional and prospective approaches
** 1-Hard dried squid or pickled radish; 2-Boiled pork meat, raw carrots, or celery; 3-Deep-fried tofu, pickled octopus, tsukemono made from Chinese cabbage, or raisins; 4-Rice, apples, fish cake, or boiled asparagus; 5-Bananas, boiled beans, canned corned beef, or wafers; 6-Unable to chew food listed in 5
Prospective approach
The univariate analyses showed that age, being female, single, or widowed, retired and unemployed, retired, and engaged with housework, retired and no longer doing housework, satisfied and dissatisfied denture users, and able to chew only softer foods at the baseline were associated with a higher FI score at the follow-up. We must highlight that having completed Grade 12, junior college, or higher (compared with those having completed Grade 9) and each additional tooth reported were associated with a lower FI score at the follow-up. The covariates that remained associated with higher FI scores at the follow-up in the multivariable analyses were age, being single, and working status (Supplementary Table C). Regarding self-reported oral diseases, being a dissatisfied denture user and able to chew food from Groups 2–6 were associated with a higher FI score at the follow-up. Each additional tooth reported was associated with a 1.5% lower FI score at the follow-up (Table 3).
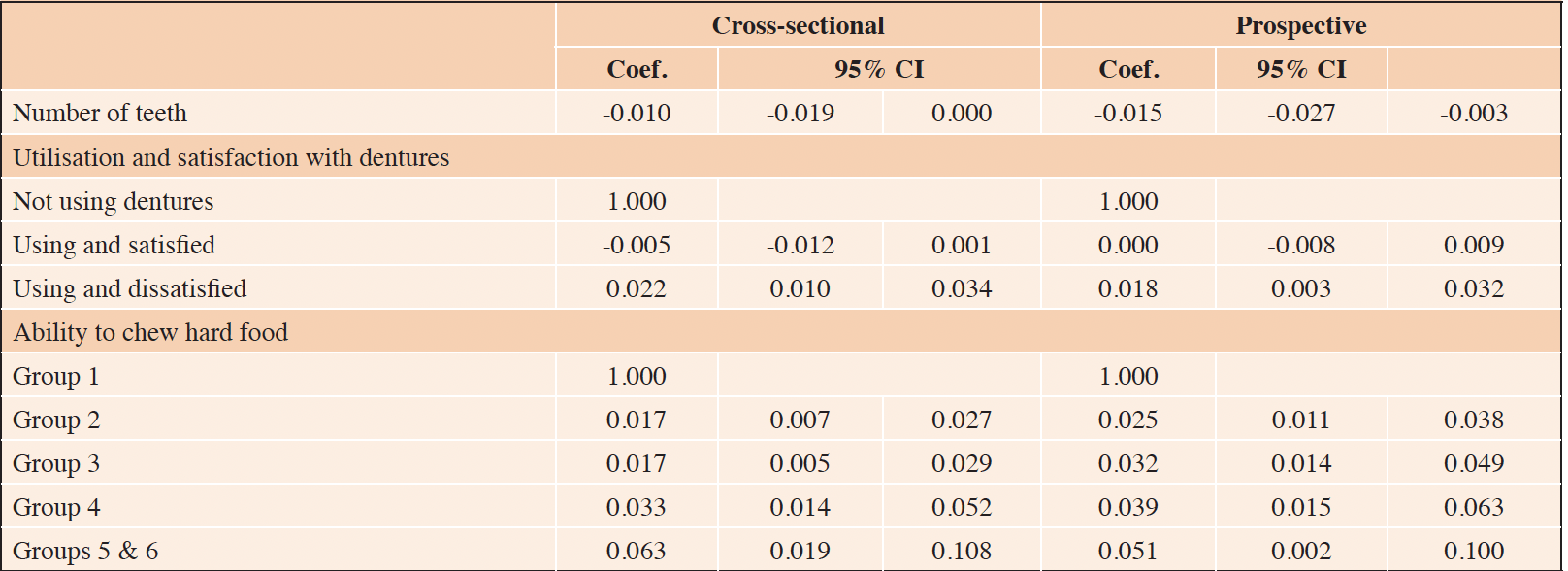
Table 3. Multivariate generalized linear regression models between self-reported oral diseases and the Frailty Index among community-dwelling older Japanese people: Cross-sectional and prospective approaches
*Covariates included in each model: Age, marital status, education background, and working status. ** 1-Hard dried squid or pickled radish; 2-Boiled pork meat, raw carrots, or celery; 3-Deep-fried tofu, pickled octopus, tsukemono made from Chinese cabbage, or raisins; 4-Rice, apples, fish cake, or boiled asparagus; 5-Bananas, boiled beans, canned corned beef, or wafers; 6-Unable to chew food listed in 5
Discussion
This study aimed to evaluate the associations between three self-reported oral diseases (number of teeth, utilization of and satisfaction with dentures, and ability to chew hard food) with the FI at the baseline and four years later among a sample of older Japanese people. We observed that each natural tooth reported at the baseline was associated with a lower FI score four years later. Being a dissatisfied denture user and having a reduced ability to chew hard food at the baseline were associated with a higher FI score at the baseline and an increased FI score four years later. These results suggest that the FI score of dissatisfied denture users and those with a reduced ability to chew increases over the years, while the increment in the FI score over time is lower for each additional natural tooth preserved.
The subjective nature of the oral diseases used in this manuscript could be a limitation of this study. Nevertheless, our results on the self-reported number of teeth and the ability to chew hard food are similar to those of studies of older Mexican and Chinese people, showing that a higher number of teeth was associated with a reduced incidence of frailty (19, 33). Our results are also consistent with a study of older Japanese people showing that a higher occlusal force and better chewing ability were associated with a lower incidence of frailty (31, 34, 35). These studies used objective oral measurements with a two- to five-year follow-up and measured frailty with the frailty phenotype. To the best of our knowledge, there is no previous study regarding self-reported utilization and satisfaction with dentures; nonetheless, this variable is similar to self-reported chewing ability, which has been previously associated with a higher incidence of frailty (35). Another study limitation is the data collection timeframe (1999–2003). However, since our results align with those of studies gathering “more recent” data, the external validity of our results is assumed.
An additional limitation related to the self-reported nature of the variables in our study is that we could not include any measurements of periodontal diseases. Despite the sound theoretical bases suggesting an association between periodontitis and frailty (41–43), few studies have reported an association between periodontal status and frailty cross-sectionally and prospectively (21, 23, 28, 33). Some studies have identified an association between periodontal disease and chronic diseases (44, 45) that are also associated with frailty (46–49); therefore, an association between periodontal status and frailty is expected (42). However, the scarce evidence on this association points to the need for additional research using currently accepted criteria for periodontal and frailty measurements and classifications for appropriate interpretation.
Our study reported a consistent association between self-reported oral diseases and frailty. This suggests that these subjective variables can be included in questionnaires used in future studies on this topic. Further investigations could use larger samples to confirm our findings and identify additional associations. The results of our self-reported independent variables may also indicate the relevance of early interventions, since the onset of these oral diseases occurs early in life, with individuals reporting no recognizable eating limitations (i.e., chewing or swallowing) as symptoms. Individuals with these oral diseases may change the components of their diet, replacing essential nutrients with something more comfortably chewed and swallowed but lower in nutritional content (Figure 2). Other studies have confirmed this (9, 27, 50–54).
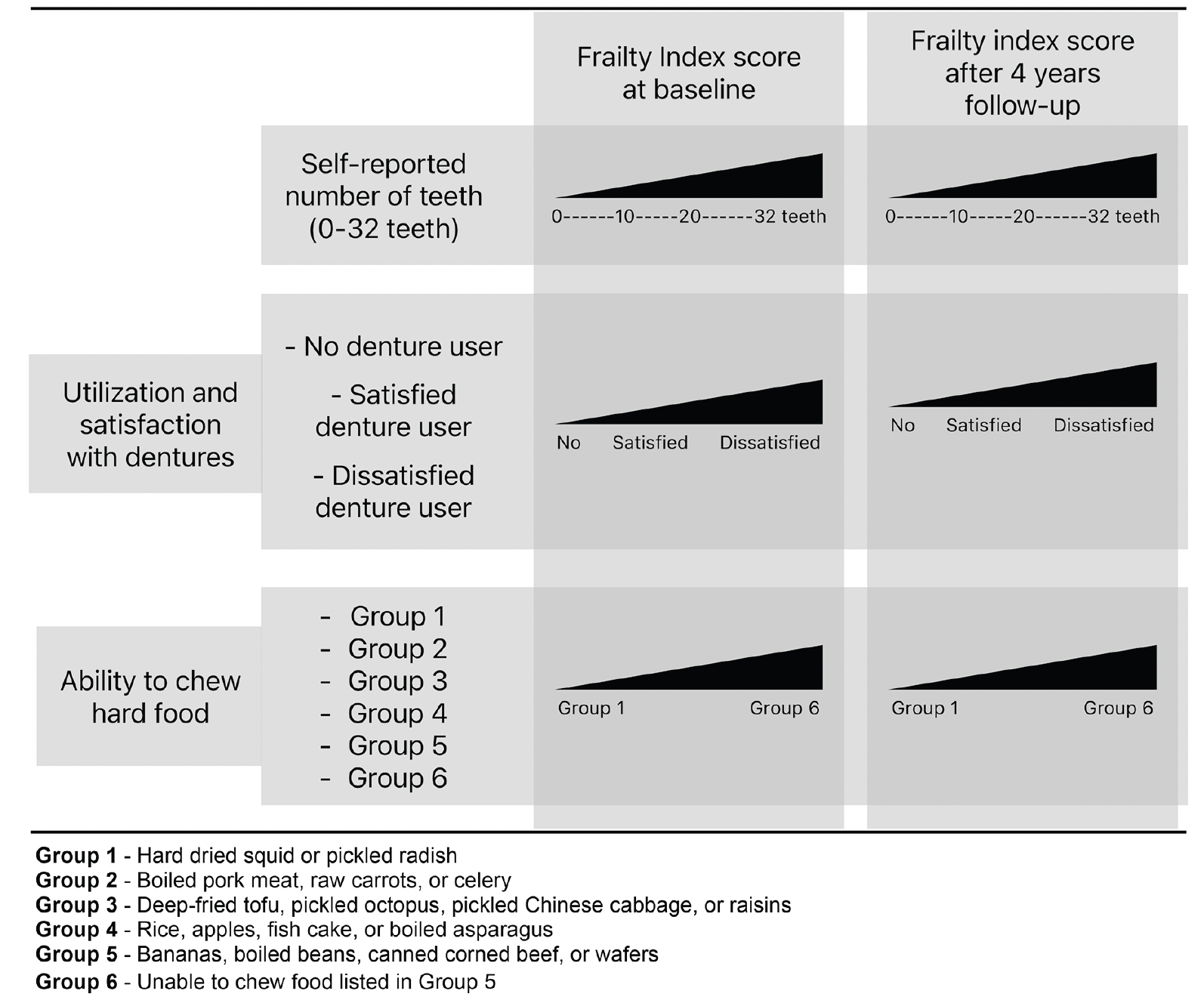
Figure 1. Conceptual model and analysis. Self-reported oral diseases associated with the Frailty Index among community-dwelling older Japanese people
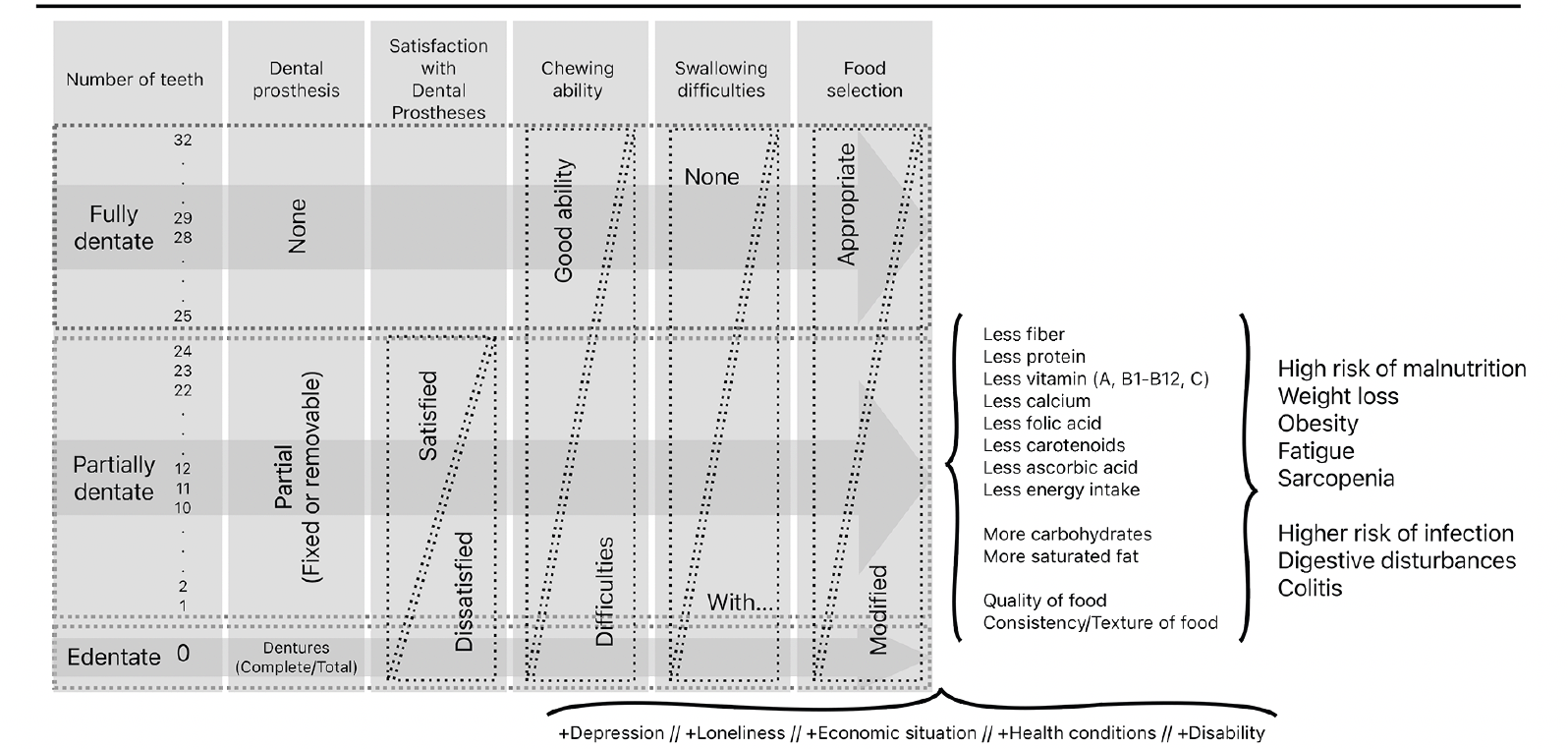
Figure 2. Process of deterioration of oral diseases leading to changes in food selection and quality with consequences
It is important to note that one study has reported an interaction between the number of teeth and diabetes (33). This finding reflects the challenge of any approach to research on older people who, over time, accumulate various health experiences that may interact with each other (17, 55–57). This is part of the rationale for developing the FI and the concept of frailty (16, 17, 55, 57, 58). Several indices claim to measure frailty (16, 55, 57–61). The frailty phenotype derived from the Cardiovascular Health Study is the most popular, but it has been criticized because it is predominantly related to muscle strength (16, 62). Most other indices have been validated by probing predictive validity and using the frailty phenotype as a reference (14). The main advantage of the frailty phenotype is its clinical utility and ease of use. However, the FI is a more comprehensive approach because it is a single measurement that considers the accumulation of deficits over an entire lifetime and provides an appropriate overview of the whole physiological system and its complexity (14, 17, 39, 56, 57, 61, 62).
Several advantages exist for prospectively identifying factors associated with frailty. Clinicians may consider these relevant factors when designing personalized treatment plans for older people. Health economists may consider the impact of oral diseases on older people’s treatment costs at the familial, institutional, and governmental levels. Our results suggest that preventive oral care could be essential for community health interventions. Similar analyses, including mortality data, could investigate whether oral diseases (objectively or subjectively measured) are related to mortality and frailty. It would also be worthwhile to examine whether improving the chewing ability of adults via dental interventions could help reduce the incidence and prevalence of frailty later in life.
Conclusion
This study shows that being a dissatisfied denture user and unable to chew hard food are associated with higher FI scores cross-sectionally and prospectively after controlling for relevant covariates. Each additional tooth is prospectively associated with a lower FI score after controlling for covariates. Our findings demonstrate that measuring self-reported oral diseases is suitable for identifying cross-sectional and prospective associations between oral health and frailty. Our results also suggest the importance of preserving oral health (i.e., preventing tooth loss, maintaining the ability to chew, and improving satisfaction with dentures) during youth and adulthood to reduce the risk of adverse health outcomes later in life.
Acknowledgments: The authors thank Luis Miguel Gutiérrez Robledo, Carmen García-Peña, and Nihon University for supporting this manuscript.
Conflict of interest: The authors declare no conflicts of interest relevant to this manuscript.
Authors’ contributions: All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. YS participated in the design and data collection of the study and the analysis and discussion of the results. IN was involved in the design and data collection of the study and discussion of the results. RAR participated in the design of the analyses and interpretation of the data. SABY contributed to the interpretation and discussion of the results. RCCP was involved in the design of the data analyses and discussion of the results.
Funding: Nihon University funded the Nihon University Japanese Longitudinal Study on Aging. The study was initiated with support from the Population Research Institute, Nihon University, while the first author of this article was a visiting research fellow at the Institute.
Ethical standards: The study followed the ethical standards in accordance with the Ethics Committee from the Nihon University and following the Helsinki declaration of 1975.
References
1. Organization GWH. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030.: Geneva: World Health Organization; 2022 November 18, 2022.
2. Benzian H, Guarnizo-Herreño CC, Kearns C, Muriithi MW, Watt RG. The WHO global strategy for oral health: an opportunity for bold action. The Lancet. 2021;398(10296):192-4. https://doi.org/10.1016/S0140-6736(21)01404-5
3. Elani HW, Batista AFM, Thomson WM, Kawachi I, Chiavegatto Filho ADP. Predictors of tooth loss: A machine learning approach. PLoS One. 2021;16(6):e0252873. https://doi.org/10.1371/journal.pone.0252873
4. Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28(6):399-406. https://doi.org/10.1034/j.1600-0528.2000.028006399.x
5. Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249-60. https://doi.org/10.1016/S0140-6736(19)31146-8
6. Schierz O, Baba K, Fueki K. Functional oral health-related quality of life impact: A systematic review in populations with tooth loss. Journal of oral rehabilitation. 2021;48(3):256-70. https://doi.org/10.1111/joor.12984
7. Jansson L, Kalkali H, Mulk Niazi F. Mortality rate and oral health – a cohort study over 44 years in the county of Stockholm. Acta Odontol Scand. 2018;76(4):299-304. https://doi.org/10.1080/00016357.2018.1423576
8. Maeda K, Mori N. Poor oral health and mortality in geriatric patients admitted to an acute hospital: an observational study. BMC geriatrics. 2020;20(1):26. https://doi.org/10.1186/s12877-020-1429-z
9. Sadamori S, Hayashi S, Hamada T. The relationships between oral status, physical and mental health, nutritional status and diet type in elderly Japanese women with dementia. Gerodontology. 2008;25(4):205-9. https://doi.org/10.1111/j.1741-2358.2008.00224.x
10. Jung YM, Shin DS. Oral health, nutrition, and oral health-related quality of life among Korean older adults. J Gerontol Nurs. 2008;34(10):28-35. https://doi.org/10.3928/00989134-20081001-09
11. Nowjack-Raymer RE, Sheiham A. Numbers of natural teeth, diet, and nutritional status in US adults. J Dent Res. 2007;86(12):1171-5. https://doi.org/10.1177/154405910708601206
12. Allen PF. Association between diet, social resources and oral health related quality of life in edentulous patients. Journal of oral rehabilitation. 2005;32(9):623-8. https://doi.org/10.1111/j.1365-2842.2005.01488.x
13. Locker D. Oral health and quality of life. Oral Health Prev Dent. 2004;2 Suppl 1:247-53.
14. Thillainadesan J, Scott IA, Le Couteur DG. Frailty, a multisystem ageing syndrome. Age Ageing. 2020;49(5):758-63. https://doi.org/10.1093/ageing/afaa112
15. Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodriguez-Manas L, Fried LP, et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J Nutr Health Aging. 2019;23(9):771-87. https://doi.org/10.1007/s12603-019-1273-z
16. Fried LP, Tangen CM, Waltson J. Frailty in older adults: evidence for a phenotype. J Gerontol. 2002;56(A):M146-M56. https://doi.org/10.1093/gerona/56.3.M146
17. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323-36. https://doi.org/10.1100/tsw.2001.58
18. Slashcheva LD, Karjalahti E, Hassett LC, Smith B, Chamberlain AM. A systematic review and gap analysis of frailty and oral health characteristics in older adults: A call for clinical translation. Gerodontology. 2021. https://doi.org/10.1111/ger.12577
19. Gu Y, Wu W, Bai J, Chen X, Chen X, Yu L, et al. Association between the number of teeth and frailty among Chinese older adults: a nationwide cross-sectional study. BMJ open. 2019;9(10):e029929. https://doi.org/10.1136/bmjopen-2019-029929
20. Hakeem F, Bernabé E, Fadel HT, Sabbah W. Association Between Oral Health and Frailty Among Older Adults in Madinah, Saudi Arabia: A Cross-Sectional Study. The journal of nutrition, health & aging. 2020. https://doi.org/10.1007/s12603-020-1419-z
21. Hakeem FF, Bernabe E, Sabbah W. Association Between Oral Health and Frailty Among American Older Adults. J Am Med Dir Assoc. 2021;22(3):559-63 e2. https://doi.org/10.1016/j.jamda.2020.07.023
22. Zhang Y, Ge M, Zhao W, Hou L, Xia X, Liu X, et al. Association between number of teeth, denture use and frailty: Findings from the West China health and aging trend study. The journal of nutrition, health & aging. 2020:1-6. https://doi.org/10.1007/s12603-020-1346-z
23. Everaars B, Jerković-Ćosić K, Bleijenberg N, de Wit NJ, van der Heijden G. Exploring Associations between Oral Health and Frailty in Community-Dwelling Older People. J Frailty Aging. 2021;10(1):56-62. https://doi.org/10.14283/jfa.2020.55
24. Castrejon-Perez RC, Wanyonyi KL, Garcia-Vazquez PE, Cruz-Hervert LP, Ramirez-Aldana R, Borges-Yanez SA. Frailty index and ten oral conditions in the Coyoacan cohort study: A cross-sectional analysis. Gerodontology. 2022. https://doi.org/10.1111/ger.12665
25. Zhang J, Xu G, Xu L. Number of Teeth and Denture Use Are Associated with Frailty among Chinese Older Adults: A Cohort Study Based on the CLHLS from 2008 to 2018. The journal of nutrition, health & aging. 2023:1-8. https://doi.org/10.1007/s12603-023-2014-x
26. Kimble R, Papacosta AO, Lennon LT, Whincup PH, Weyant RJ, Mathers JC, et al. The Relationship of Oral Health with Progression of Physical Frailty among Older Adults: A Longitudinal Study Composed of Two Cohorts of Older Adults from the United Kingdom and United States. J Am Med Dir Assoc. 2023;24(4):468-74 e3. https://doi.org/10.1016/j.jamda.2022.11.022
27. Kimble R, Papacosta AO, Lennon LT, Whincup PH, Weyant RJ, Mathers JC, et al. The Relationships of Dentition, Use of Dental Prothesis and Oral Health Problems with Frailty, Disability and Diet Quality: Results from Population-Based Studies of Older Adults from the UK and USA. J Nutr Health Aging. 2023;27(8):663-72. https://doi.org/10.1007/s12603-023-1951-8
28. Castrejon-Pérez RC, Borges-Yánez SA, Gutierrez-Robledo LM, Avila-Funes JA. Oral health conditions and frailty in Mexican community-dwelling elderly: a cross sectional analysis. BMC Public Health. 2012;12:773. https://doi.org/10.1186/1471-2458-12-773
29. Hakeem FF, Bernabe E, Sabbah W. Association between oral health and frailty: A systematic review of longitudinal studies. Gerodontology. 2019. https://doi.org/10.1111/ger.12406
30. Torres LH, Tellez M, Hilgert JB, Hugo FN, de Sousa MD, Ismail AI. Frailty, Frailty Components, and Oral Health: A Systematic Review. J Am Geriatr Soc. 2015;63(12):2555-62. https://doi.org/10.1111/jgs.13826
31. Iwasaki M, Yoshihara A, Sato M, Minagawa K, Shimada M, Nishimuta M, et al. Dentition status and frailty in community-dwelling older adults: A 5-year prospective cohort study. Geriatr Gerontol Int. 2018;18(2):256-62. https://doi.org/10.1111/ggi.13170
32. Ramsay SE, Papachristou E, Watt RG, Tsakos G, Lennon LT, Papacosta AO, et al. Influence of Poor Oral Health on Physical Frailty: A Population-Based Cohort Study of Older British Men. J Am Geriatr Soc. 2018;66(3):473-9. https://doi.org/10.1111/jgs.15175
33. Castrejón-Pérez RC, Jiménez-Corona A, Bernabé E, Villa-Romero AR, Arrivé E, Dartigues JF, et al. Oral Disease and 3-Year Incidence of Frailty in Mexican Older Adults. J Gerontol A Biol Sci Med Sci. 2017;72(7):951-7. https://doi.org/10.1093/gerona/glw201
34. Horibe Y, Ueda T, Watanabe Y, Motokawa K, Edahiro A, Hirano H, et al. A 2-year longitudinal study of the relationship between masticatory function and progression to frailty or pre-frailty among community-dwelling Japanese aged 65 and older. Journal of oral rehabilitation. 2018;45(11):864-70. https://doi.org/10.1111/joor.12700
35. Iwasaki M, Yoshihara A, Sato N, Sato M, Minagawa K, Shimada M, et al. A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. Journal of oral rehabilitation. 2018;45(1):17-24. https://doi.org/10.1111/joor.12578
36. Chan A, Zimmer Z, Saito Y. Gender differentials in disability and mortality transitions: the case of older adults in Japan. J Aging Health. 2011;23(8):1285-308. https://doi.org/10.1177/0898264311408417
37. Nasu I, Saito Y. [Active life expectancy for elderly Japanese by chewing ability]. [Nihon koshu eisei zasshi] Japanese journal of public health. 2006;53(6):411-23.
38. NUJLSOA. Nihon University Longitudinal Study of Aging: Nihon University Longitudinal Study of Aging; 2023 [updated 2023; cited 2023 9/3/23]. Available from: https://gero.usc.edu/cbph/nujlsoa/overview/.
39. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC geriatrics. 2008;8:24. https://doi.org/10.1186/1471-2318-8-24
40. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Archives of gerontology and geriatrics. 2015;60(3):464-70. https://doi.org/10.1016/j.archger.2015.01.016
41. Yan GLK, Tan MN, Wong ML, Tay CM, Allen PF. Functional Dentition, Chronic Periodontal Disease and Frailty in Older Adults-A Narrative Review. Int J Environ Res Public Health. 2022;20(1). https://doi.org/10.3390/ijerph20010502
42. Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontol 2000. 2021;87(1):143-56. https://doi.org/10.1111/prd.12380
43. Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol. 2020;47(3):268-88. https://doi.org/10.1111/jcpe.13189
44. Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Periodontol. 2013;84(4 Suppl):S70-84. https://doi.org/10.1902/jop.2013.134008
45. Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(4 Suppl):S106-12. https://doi.org/10.1902/jop.2013.1340011
46. Ramsay SE, Arianayagam DS, Whincup PH, Lennon LT, Cryer J, Papacosta AO, et al. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart. 2015;101(8):616-22. https://doi.org/10.1136/heartjnl-2014-306472
47. Woo J. Nutrition and Frailty. J Nutr Health Aging. 2018;22(9):1025-7. https://doi.org/10.1007/s12603-018-1120-7
48. Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism. 2017;68:64-76. https://doi.org/10.1016/j.metabol.2016.12.005
49. Castrejon-Perez RC, Aguilar-Salinas CA, Gutierrez-Robledo LM, Cesari M, Perez-Zepeda MU. Frailty, diabetes, and the convergence of chronic disease in an age-related condition: a population-based nationwide cross-sectional analysis of the Mexican nutrition and health survey. Aging Clin Exp Res. 2018;30(8):935-41. https://doi.org/10.1007/s40520-017-0852-2
50. Figueredo OMC, Câmara-Souza MB, Carletti TM, Rodrigues Garcia RCM. Chewing ability and oral health-related quality of life in frail elders after new complete dentures insertion: A paired controlled clinical trial. Special care in dentistry : official publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry. 2020;40(2):168-74. https://doi.org/10.1111/scd.12448
51. Moynihan PJ. The relationship between nutrition and systemic and oral well-being in older people. J Am Dent Assoc. 2007;138(4):493-7. https://doi.org/10.14219/jada.archive.2007.0201
52. Nakamura M, Ojima T, Nakade M, Ohtsuka R, Yamamoto T, Suzuki K, et al. Poor Oral Health and Diet in Relation to Weight Loss, Stable Underweight, and Obesity in Community-Dwelling Older Adults: A Cross-Sectional Study From the JAGES 2010 Project. J Epidemiol. 2016;26(6):322-9. https://doi.org/10.2188/jea.JE20150144
53. Kusama T, Takeuchi K, Kiuchi S, Aida J, Hikichi H, Sasaki S, et al. Dental prosthesis use is associated with higher protein intake among older adults with tooth loss. Journal of oral rehabilitation. 2023;50(11):1229-38. https://doi.org/10.1111/joor.13554
54. Chan AKY, Tsang YC, Jiang CM, Leung KCM, Lo ECM, Chu CH. Diet, Nutrition, and Oral Health in Older Adults: A Review of the Literature. Dent J (Basel). 2023;11(9). https://doi.org/10.3390/dj11090222
55. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123(11):1457-60. https://doi.org/10.1016/S0047-6374(02)00082-9
56. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The accumulation of deficits with age and possible invariants of aging. ScientificWorldJournal. 2002;2:1816-22. https://doi.org/10.1100/tsw.2002.861
57. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-7. https://doi.org/10.1093/gerona/62.7.722
58. Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev. 2004;125(7):517-9. https://doi.org/10.1016/j.mad.2004.05.003
59. Amblas-Novellas J, Espaulella-Panicot J, Inzitari M, Rexach L, Fontecha B, Romero-Ortuno R. [The challenge of clinical complexity in the 21st century: Could frailty indexes be the answer?]. Rev Esp Geriatr Gerontol. 2017;52(3):159-66. https://doi.org/10.1016/j.regg.2016.07.005
60. Guaraldi G, Malagoli A, Theou O, Brothers TD, Wallace L, Torelli R, et al. Correlates of frailty phenotype and frailty index and their associations with clinical outcomes. HIV Med. 2017;18(10):764-71. https://doi.org/10.1111/hiv.12527
61. Rockwood K, Blodgett JM, Theou O, Sun MH, Feridooni HA, Mitnitski A, et al. A Frailty Index Based On Deficit Accumulation Quantifies Mortality Risk in Humans and in Mice. Sci Rep. 2017;7:43068. https://doi.org/10.1038/srep43068
62. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1-15. https://doi.org/10.1016/j.cger.2010.08.009
© Serdi 2024
