C. Iwasaka1,2, H. Nanri1,2,3, M. Hara2, Y. Nishida2, C. Shimanoe4, Y. Yamada1,3, T. Furukawa2,5, Y. Higaki6,
Y. Momozawa7, M. Nakatochi8, K. Wakai9, K. Matsuo10, K. Tanaka2
1. Department of Physical Activity Research, National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan; 2. Department of Preventive Medicine, Faculty of Medicine, Saga University, Saga, Japan; 3. Laboratory of Gut Microbiome for Health, Microbial Research Center for Health and Medicine, National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan; 4. Department of Pharmacy, Saga University Hospital, Saga, Japan; 5. Clinical Research Center, Saga University Hospital, Saga, Japan; 6. Laboratory of Exercise Physiology, Faculty of Sports and Health Science, Fukuoka University, Fukuoka, Japan; 7. Laboratory for Genotyping Development, RIKEN Center for Integrative Medical Sciences, Kanagawa, Japan; 8. Public Health Informatics Unit, Department of Integrated Health Sciences, Nagoya University Graduate School of Medicine, Aichi, Japan; 9. Department of Preventive Medicine, Nagoya University Graduate School of Medicine, Aichi, Japan; 10. Division of Cancer Epidemiology and Prevention, Aichi Cancer Center, Aichi, Japan
Corresponding Author: Chiharu Iwasaka, PhD, Department of Physical Activity Research, National Institutes of Biomedical Innovation, Health and Nutrition, KENTO Innovation Park NK Building, 3-17, Senriokashinmachi, Settsu, Osaka, 566-0002, Japan, E-mail: ciwasaka@nibiohn.go.jp, Tel.: +81-6-6384-1120; Hinako Nanri, PhD, Department of Physical Activity Research, National Institutes of Biomedical Innovation, Health and Nutrition, KENTO Innovation Park NK Building, 3-17, Senriokashinmachi, Settsu, Osaka, 566-0002, Japan, E-mail: hnanri@nibiohn.go.jp, Tel.: +81-6-6384-1120
J Frailty Aging 2024;in press
Published online March 19, 2024, http://dx.doi.org/10.14283/jfa.2024.3
Abstract
BACKGROUND: Recent studies have suggested the potential benefits of habitual coffee and green tea consumption on skeletal muscle health. However, it remains unclear whether these benefits are modified by genetic factors, particularly the alpha-actinin-3 (ACTN3) genotype, which is associated with the skeletal muscle phenotype. This study aimed to investigate the interaction between habitual coffee or green tea consumption and the ACTN3 genotype in association with skeletal muscle mass (SMM) and strength.
METHODS: This cross-sectional study was conducted on 1,023 Japanese middle-aged and older adults (619 females, aged 45–74 years) living in the community. SMM was gauged using a bioelectrical impedance spectroscopy device, and handgrip strength (HGS) was used to measure muscle strength. The ACTN3 genotype (RR, RX, and XX) was determined from blood samples. Sex-specific linear regression models were used to analyze the interactions between coffee or green tea consumption and the ACTN3 genotype in association with SMM and HGS.
RESULTS: In females, a significant interaction was observed between green tea consumption and the ACTN3 genotype in association with HGS (P interaction < 0.05). Furthermore, stratified analysis revealed a positive association between green tea consumption and HGS, specifically in females with the ACTN3 XX genotype (P trend < 0.05). In males, no significant interactions were observed between coffee or green tea consumption and the ACTN3 genotype in association with SMM or HGS (P interaction > 0.05).
CONCLUSION: Our findings suggest that the skeletal muscle strength benefits associated with habitual green tea consumption may be contingent upon sex and the ACTN3 genotype.
Key words: Coffee, tea, muscle strength, ACTN3 protein, human.
Abbreviations: ACTN3: alpha-actinin-3; SMM: skeletal muscle mass; HGS: handgrip strength; FFQ: food frequency questionnaire; FMI: fat mass index; PAL: physical activity level; SMI: skeletal muscle mass index.
Introduction
Frailty and sarcopenia, conditions frequently associated with an elevated risk of falls, hospitalization, and mortality, are becoming pressing health concerns in rapidly aging populations (1, 2). Skeletal muscle aging is a cardinal determinant of these conditions, emphasizing the importance of understanding the mechanisms and interventions to curb their onset and progression (3, 4). Additionally, the interaction between genetic predispositions and lifestyle choices may offer deeper insights into these mechanisms, underscoring the need to explore these interactions to develop targeted preventive strategies (5, 6).
Genetic factors accounting for muscle strength and skeletal muscle mass (SMM) variability have been reported to be 32–67% and 46–76%, respectively (5). The alpha-actinin-3 (ACTN3) genotype, which contributes to the skeletal muscle phenotype, encodes α-actinin-3, a protein expressed in fast-twitch muscle fibers (7). This gene polymorphism has been reported to affect lower SMM and its function. X-allele holders are characterized by lower muscle mass and strength than R-allele holders and are considered a potential genetic risk factor for the development of sarcopenia (7).
Emerging evidence has suggested the habitual consumption of green tea and coffee as a potential modifiable factor for the prevention of frailty and/or sarcopenia (8-13). Although the biological mechanisms by which habitual consumption of green tea and coffee affects SMM and skeletal muscle function have not been fully elucidated, it has been suggested that various antioxidant and anti-inflammatory pathways induce autophagy and differentiation of myogenic stem cells because of the active ingredients in green tea and coffee, polyphenols (tea catechins and chlorogenic acid) and caffeine, and may prevent age-related decline in SMM and muscle strength (13-16). However, how genetic factors modulate the associations between habitual green tea and coffee consumption and skeletal muscle has not yet been investigated.
In this study, we examined the potential interaction between habitual coffee or green tea consumption and the ACTN3 genotype in association with skeletal muscle strength and mass. We hypothesized that the benefits of green tea and coffee consumption vary depending on an individual’s ACTN3 genotype.
Methods
Study design and population
This cross-sectional study was conducted using secondary survey data from the Japan Multi-institutional Collaborative Cohort Study in the Saga Region (Saga J-MICC Study) (17, 18). Details of the Saga J-MICC study have been reported previously (17, 18). Briefly, the J-MICC study was initiated in 2005 by 10 research groups in Japan to investigate the interaction between genes and the environment in lifestyle-related diseases, such as cancer (19). The first survey in the Saga region included 12,078 participants; the second survey, conducted 5 years later, included 8,454 of the 12,078 participants. Skeletal muscle assessment, including handgrip strength (HGS) and SMM measurements, was newly added to the secondary survey (11, 20, 21). Genotype data were available for the analysis of a subset of secondary study participants (random selection) from the Saga region (22). Therefore, 1,350 participants in the secondary survey for whom genotype data were available for analysis were considered for this study. Out of 1,350 participants, 327 were excluded from the analysis for the following reasons: missing data on coffee consumption (n = 1), green tea consumption (n = 1), SMI (n = 15), HGS (n = 4), and covariates such as FMI (n = 15) and PAL (n = 44). Moreover, participants with a history of cancer (n = 107), cardiovascular disease (n = 87), diabetes (n = 118), liver disease (n = 18), and renal failure (n = 3) were excluded to reduce comorbid influences on the skeletal muscle phenotype since these conditions can independently affect muscle health. Consequently, the final analysis encompassed 1,023 individuals (619 females).
The research protocol was approved by the ethics committees of the Saga University Faculty of Medicine (approval no. 17-11) and Nagoya University Graduate School of Medicine (approval no. 253). The purpose, content, and conditions of the study were explained in writing and orally, and the participants provided written informed consent to participate in the study.
Assessment of habitual coffee and green tea consumption
The habitual consumption of coffee or green tea over the past year was assessed using a validated Japanese Food Frequency Questionnaire (FFQ) (23). This FFQ was validated against 3-day weighted dietary records for energy and 26 specific nutrients, yielding correlation coefficients primarily within the range of 0.4 to 0.6 (23). A subsequent validation study evaluated the accuracy of FFQ regarding food group intake, and the identified correlation coefficients ranged from 0.17 to 0.76 in males and 0.23 to 0.77 in females (24). Specifically for coffee and green tea, the correlation coefficients were 0.56 and 0.52, respectively, for males and 0.56 and 0.51, respectively, for females (24). The frequency of coffee and green tea consumption was evaluated as follows: almost none, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, 1 time/day, 2 times/day, and >3 times/day. The frequency of coffee and green tea consumption was classified into four categories (almost none, <1 cup/day, 1–2 cups/day, and >3 cups/day) based on previous studies (11, 25).
Assessment of SMM and HGS
The SMM was gauged using a multifrequency bioelectrical impedance spectroscopy device (MLT-30, SK Medical, Electronics Co., Ltd., Shiga, Japan) (11, 20, 21, 26). Individuals with artificial joints or cardiac pacemakers were excluded from measurements. SMM measurements were conducted by placing electrodes on the right dorsal hand and right dorsal foot, and whole-body SMM was determined using specialized software accompanying MLT-30. The software computed the SMM with reference to the values measured using magnetic resonance imaging (27). In alignment with prior research, the SMM index (SMI, kg/m2) was derived by adjusting SMM against height squared (28).
To determine the HGS values of the left and right hands, a Smedley-type HGS dynamometer (Grip-D, T.K.K. 5401; Takei Scientific Instruments, Niigata, Japan) was used. The participants had their HGS gauged once for each hand while standing and with their elbows fully extended. For analytical purposes, a greater value, whether from the left or right hand, was chosen (29).
Determination of the ACTN3 genotype
Buffy coat fractions were prepared from blood samples and stored at –80 °C at the central office of the J-MICC Study. DNA was extracted from all buffy coat fractions using the BioRobot M48 Workstation (Qiagen Group) at the central office. Genotyping was conducted at the RIKEN Center for Integrative Medicine Sciences using the HumanOmniExpressExome-8 v1.2 BeadChip array (Illumina Inc.). After quality control (30), we extracted data from the ACTN3 variant (rs1815739), which was directly genotyped. The C and T alleles for rs1815739 corresponded to arginine (R) and the stop codon (X), respectively (31). Accordingly, we used the following designations: homozygous fully functional RR genotype, heterozygous intermediate RX genotype, and homozygous low-functional XX genotype (32).
Other covariates
The covariates required for analysis were selected based on a previous study (11). Age was calculated from the date of birth and date of face-to-face measurements. Data on demographics and lifestyle, including sex, smoking status, alcohol consumption, and menopausal status, were gathered using self-reported questionnaires. Data regarding smoking habits included current smoking presence/absence/quitting, when they quit, the number of cigarettes smoked per day, and the age at which they started smoking. Data regarding drinking habits (alcohol consumption) included whether they had a drinking habit (yes, if they consumed alcohol at least once a month)/never/stopped; when they stopped, the age at which they started consuming alcohol habitually, and the type and amount of alcohol that they consumed. Based on this information, smoking and alcohol consumption statuses were classified into three categories (never, former, and current). Fat mass was estimated using the same bioelectrical impedance spectroscopy device used to measure SMM, and the fat mass index (FMI, kg/m2) was calculated by dividing the fat mass by the square of height. Based on the FFQ, total energy intake (kcal/day) and protein intake (g/day) were calculated. Physical activity level (PAL) was measured using a validated accelerometer (Lifecorder, Suzuken, Japan) (22). The individuals were directed to attach the accelerometer to their belt or waistband, aligned with the midline of either thigh, throughout their awake hours (excluding bathing or swimming times) over a span of 10 days (33). After the measurement period, the accelerometers were returned via post. To mitigate the impact of alterations in PALs due to the initial adaptation to the accelerometer, the data recorded during the initial 3 days were not included in the analysis. Only the data recorded over the subsequent 7 days, wherein the device was worn for a minimum of 8 h per day, were considered for analysis (33). If the number of effective days of 8-h wear was <3, the PAL data were considered as missing values. PAL was derived by dividing the total energy output, as captured by the accelerometer, by the basal metabolic rate (33).
Statistical analysis
Genotype distributions were tested for Hardy–Weinberg equilibrium using the chi-square test. The demographic characteristics of the participants were compared based on sex and the ACTN3 genotype. Independent t-tests (continuous variables), chi-square tests (categorical variables), and Fisher’s exact tests (categorical variables) were used for sex-based comparisons. One-way analysis of variance (continuous variables), chi-square test (categorical variables), and Fisher’s exact test (categorical variables) were used for ACTN3 genotype-based comparisons.
An interaction test based on a general linear model was performed using coffee/green tea consumption (almost none, <1 cup/day, 1–2 cups/day, and >3 cups/day; ordinal) × ACTN3 genotype (RR, RX, and XX; ordinal) as the interaction term and skeletal muscle variables (i.e., SMI [continuous] and HGS [continuous]) as the outcome variables. Potential confounders included age (continuous), FMI (continuous), smoking status (never, former, and current; categorical), alcohol consumption status (never, former, and current; categorical), PAL (continuous), total energy intake (continuous), protein intake (continuous), postmenopausal status (females only; yes or no; categorical), and coffee/green tea consumption (almost none, <1 cup/day, 1–2 cups/day, and >3 cups/day; categorical; either one not an exposure variable), following previous studies (11).
Finally, a stratified analysis of the association between coffee/green tea consumption and skeletal muscle variables, stratified by the ACTN3 genotype was performed. The covariates included the same variables as above, and least-squares means, and 95% confidence intervals were calculated. Trend P-values based on linear regression models were calculated with habitual coffee or green tea consumption as the ordinal variable. All statistical analyses were performed using R version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria), with a significance level set at <5%.
Results
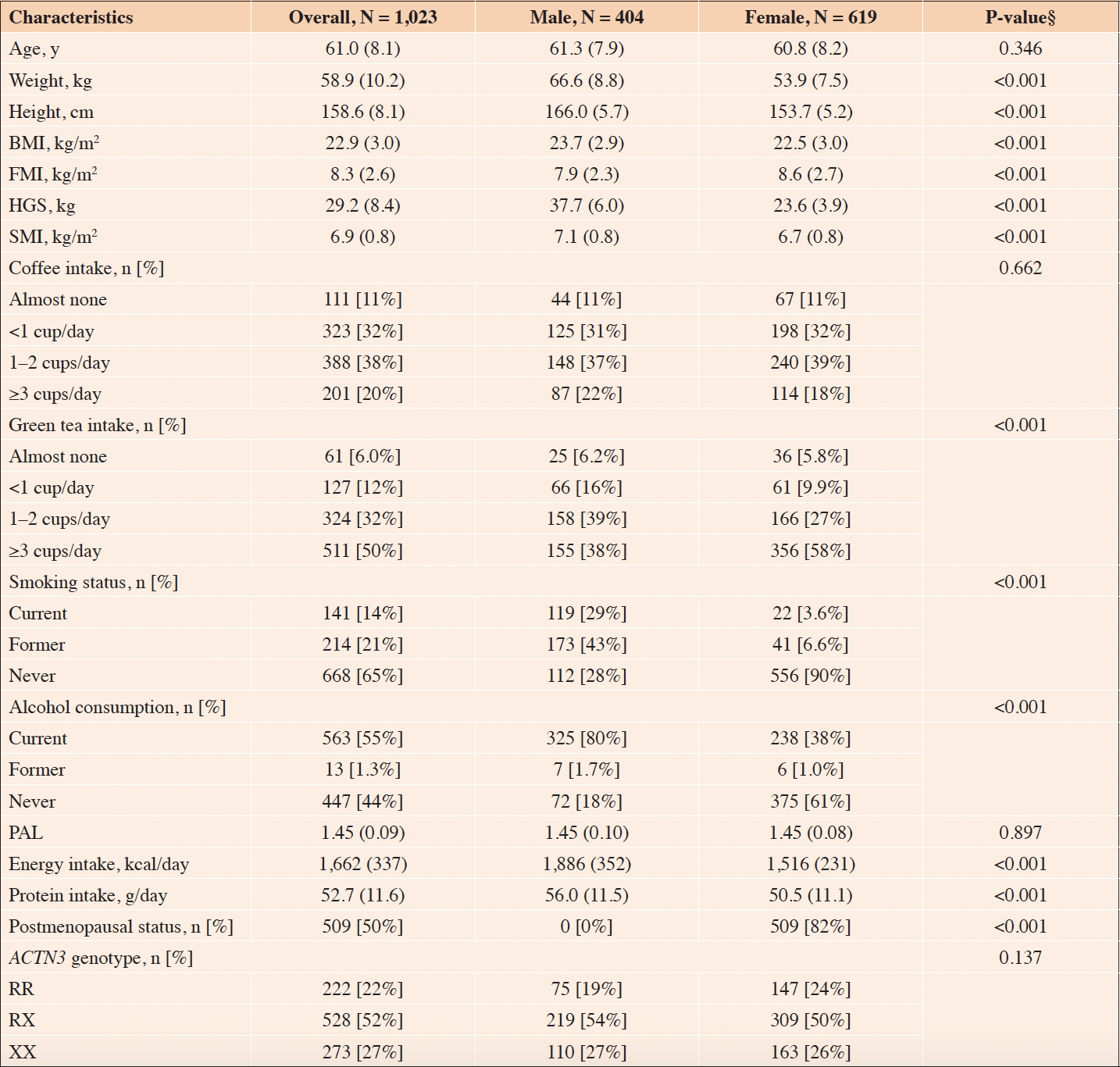
The ACTN3 genotype frequencies were in Hardy–Weinberg equilibrium (P = 0.29). Table 1 presents the demographic characteristics stratified by the sex of the participants. Significant differences were observed in all variables, except for age, coffee intake, PAL, and the ACTN3 genotype (P < 0.05).
Continuous variable, mean (standard deviation); Categorical variable, number [percent-age]. BMI, body mass index; FMI, fat mass index; HGS, handgrip strength; SMI, skeletal muscle mass index; PAL, physical activity level; ACTN3, alpha-actinin-3; RR, homozy-gous fully functional genotype; RX, heterozygous intermediate genotype; XX, homozy-gous low functional genotype. § Compared by sex.
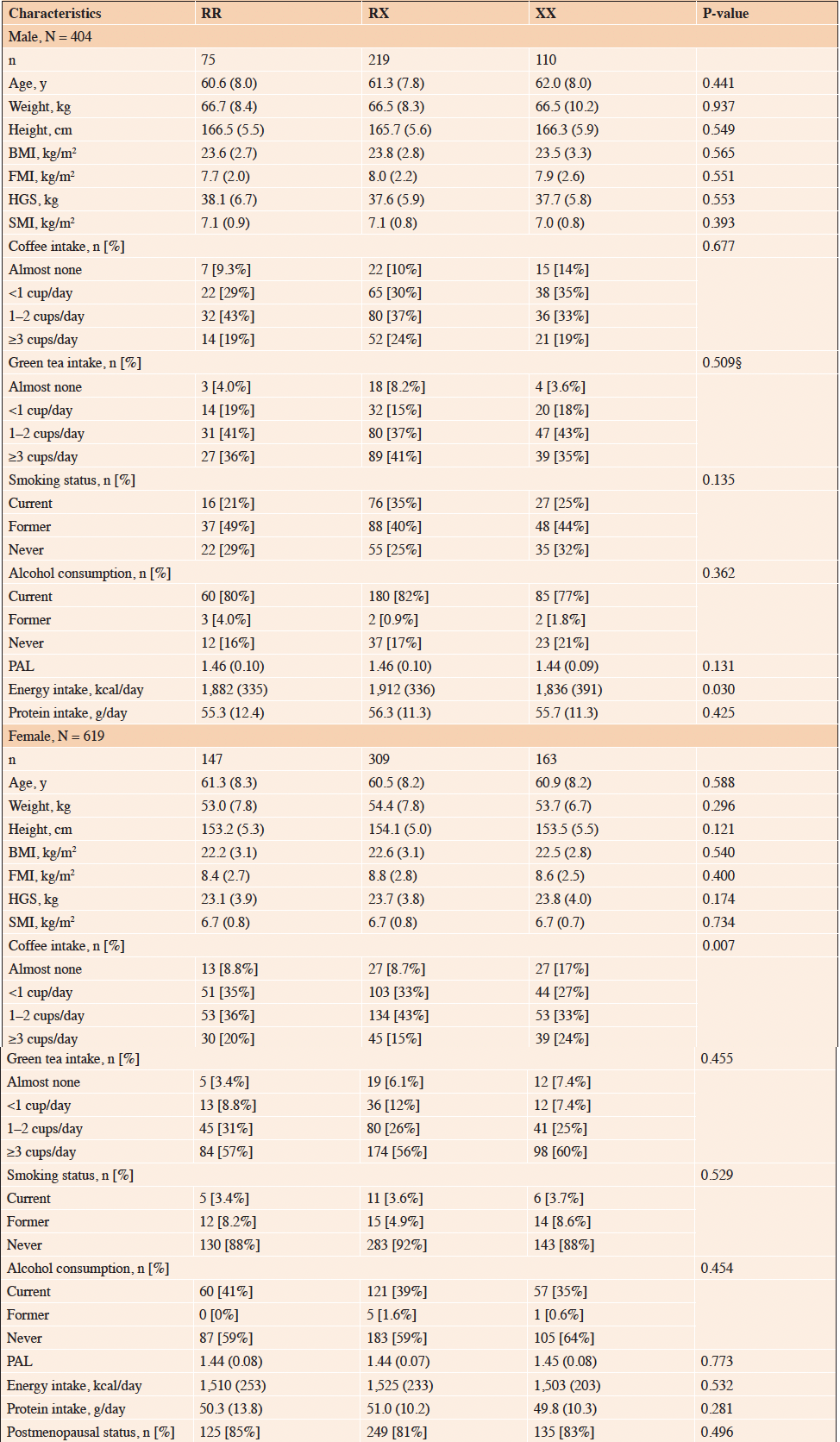
Table 2 presents demographic characteristics stratified by ACTN3 genotype. In males, there was a significant difference in energy intake (P < 0.05), while in females, a significant difference was observed in coffee consumption (P < 0.05).
Multivariate linear regression analyses showed no significant interactions between coffee consumption and the ACTN3 genotype associated with SMI (males, P for interaction = 0.43; females, P for interaction = 0.44) and HGS (males, P for interaction = 0.64; females, P for interaction = 0.46) in males and females. Similarly, no significant interactions were observed between green tea intake and the ACTN3 genotype associated with SMI in both males and females (males, P for interaction = 0.73; females, P for interaction = 0.49). However, a significant interaction was observed between green tea consumption and the ACTN3 genotype associated with HGS, exclusively in females (males, P for interaction = 0.22; females, P for interaction = 0.02).
Continuous variable, mean (standard deviation); Categorical variable, number [percentage]. BMI, body mass index; FMI, fat mass index; HGS, handgrip strength; SMI, skeletal muscle mass index; PAL, physical activity level; ACTN3, alpha-actinin-3; RR, homozygous fully functional genotype; RX, heterozygous intermediate genotype; XX, homozygous low functional genotype. § Fisher’s exact test.
Table 3 presents the stratified analysis of the association between coffee consumption and SMI or HGS, stratified by the ACTN3 genotype. Similarly, the stratified analysis for the association between green tea consumption and SMI or HGS, stratified by the ACTN3 genotype, is presented in Table 4. The analysis revealed a significant positive association between green tea consumption and HGS, exclusively in females with the ACTN3 XX genotype (Table 4, P for trend = 0.02). In contrast, no significant associations were observed between coffee consumption and SMI or HGS in females stratified by the ACTN3 genotype (Table 3, P for trend > 0.05), and no significant associations were observed between coffee and green tea consumption and SMI or HGS in males stratified by the ACTN3 genotype (Tables 3 and 4, P for trend > 0.05).
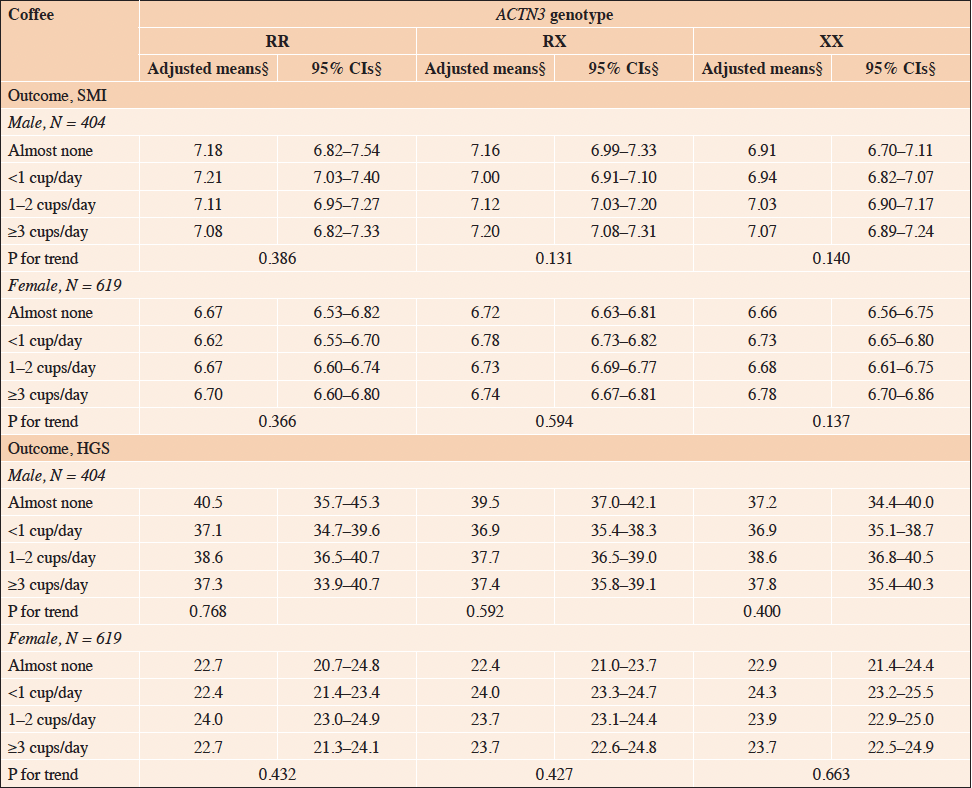
Table 3. Association of ACTN3 genotype-stratified SMI and HGS with coffee consumption: adjusted mean and 95% confidence intervals
SMI, skeletal muscle mass index; HGS, handgrip strength; ACTN3, alpha-actinin-3; RR, homozygous fully functional genotype; RX, heterozygous intermediate genotype; XX, homozygous low functional genotype; CI, confidence interval. § Adjusted means and 95% confidence intervals are adjusted for the following covariates: age, fat mass index, smoking status, alcohol consumption, total energy intake, protein intake, green tea consumption, physical activity level, and postmenopausal status (females only).
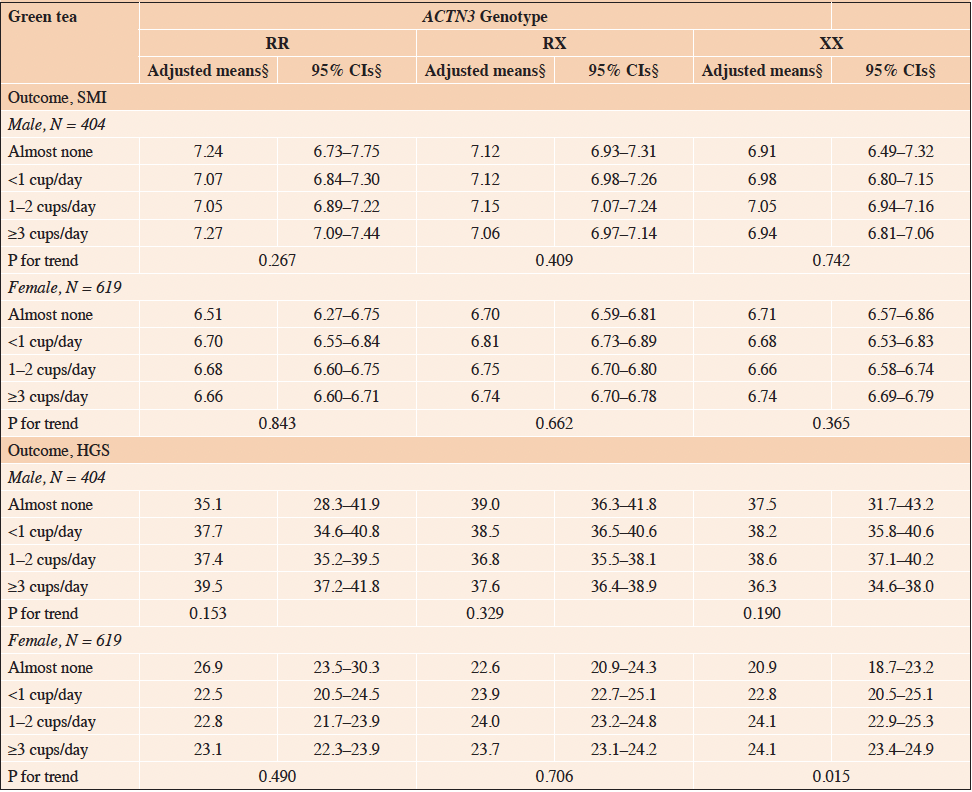
Table 4. Association of ACTN3 genotype-stratified SMI and HGS with green tea consumption: adjusted mean and 95% confidence intervals
SMI, skeletal muscle mass index; HGS, handgrip strength; ACTN3, alpha-actinin-3; RR, homozygous fully functional genotype; RX, heterozygous intermediate genotype; XX, homozygous low functional genotype; CI, confidence interval. § Adjusted means and 95% confidence intervals are adjusted for the following covariates: age, fat mass index, smoking status, alcohol consumption, total energy intake, protein intake, coffee consumption, physical activity level, and postmenopausal status (females only).
Discussion
We hypothesized that the efficacy of habitual coffee/green tea consumption on SMM and skeletal muscle strength would differ depending on the ACTN3 genotype. Our results showed a significant interaction between habitual green tea consumption and ACTN3 genotype in association with HGS in female participants. Stratified analysis showed that the positive association between habitual green tea consumption and HGS was observed only for the ACTN3 XX genotype in females. The results of this study suggest that the benefits of habitual green tea consumption on skeletal muscle strength may vary depending on the ACTN3 genotype.
In this study, we observed a significant interaction between green tea consumption and the ACTN3 genotype associated with HGS in females only. This association is difficult to interpret but given the results that allowed for a sex-specific association, it may suggest the involvement of sex hormones. Significant components of green tea, such as catechins, exhibit estrogenic activity (34). The skeletal muscle is the predominant tissue in which estrogen receptors are located (35). Estrogen deficiency in females can induce myosin dysfunction and hinder muscle regeneration, consequently leading to muscle weakness (36). Moreover, a meta-analysis indicated that estrogen-based hormone therapy may confer benefits in terms of skeletal muscle strength in postmenopausal females (35). In this study, 82% of the female participants were postmenopausal, and we observed a significant positive association between green tea consumption and HGS only in the ACTN3 XX genotype of postmenopausal females (Supplementary Table 1). Thus, it is conceivable that the regular consumption of green tea could trigger estrogen activation and mitigate age-associated muscle weakness in postmenopausal females with declining estrogen levels. In addition, carriers of the X allele of the ACTN3 gene are usually characterized by lower muscle strength, performance, and SMM than R allele carriers, indicating that they may be more susceptible to exercise-induced muscle damage (37). Repeated skeletal muscle damage and inadequate repair capacity contribute to muscle weakness in the older adults (36, 38). Given estrogen’s role in muscle repair (36), it could be speculated that individuals with the ACTN3 XX genotype, who may be predisposed to muscle damage, could potentially experience some muscle recovery benefits from habitual green tea consumption. Nonetheless, the current body of evidence remains preliminary. Comprehensive investigations are essential to unravel the potential advantages of green tea, particularly those concerning the ACTN3 XX genotype.
Habitual coffee consumption showed no significant interaction with the ACTN3 genotype associated with SMI or HGS in either sex. These results suggest that the association between habitual coffee consumption and both SMM and muscle strength is not modified by the ACTN3 genotype. While previous observational studies have found a beneficial association between habitual coffee consumption and SMM or sarcopenia (11-13, 39), our analysis found no such association with any ACTN3 genotype. This discrepancy may be attributed to factors not evaluated in this study, such as the addition of sugar or milk to coffee, or low power due to insufficient sample size. Therefore, further research is necessary to clarify the interplay between genetic factors, skeletal muscle phenotypes, and coffee consumption, including the addition of sugar and milk.
Our use of a FFQ in this study meant we couldn’t quantify potentially beneficial compounds like caffeine, tea catechins, chlorogenic acid, and caffeic acid found in green tea and coffee for skeletal muscle. Caffeine is widely recognized for its ergogenic effects, enhancing endurance, power, and muscle strength (40). Catechins could prevent, mitigate, and treat muscle-related disorders (14). Similarly, chlorogenic acid, and its metabolite caffeic acid, through studies in animal models, have been shown to improve muscle strength and glucose uptake in skeletal muscles (41, 42), suggesting their potential as effective compounds for muscle health. However, our study’s design limited our ability to directly investigate the interactions between these bioactive components and the ACTN3 genotype. Therefore, we are unable to pinpoint which specific compounds, including tea catechins which we have considered as potentially impactful, might have contributed to the observed interaction between habitual green tea consumption and HGS in females in association to the ACTN3 genotype. Future research that can quantify the intake of these compounds and explore their direct effects on muscle health and genetic interactions will be critical to deepening our understanding of these complex relationships.
In our study, we did not observe the general characteristics of low SMM, and low muscle strength compared to ACTN3 X allele carriers and R allele carriers. Several factors may contribute to this discrepancy. SMM was estimated using bioelectrical impedance analysis, which, compared to gold-standard methods such as magnetic resonance imaging or computed tomography, may have limited ability to detect minor differences in SMM, potentially leading to prediction errors (43). Skeletal muscle strength assessment in our study was based solely on HGS. While HGS is commonly used as a proxy for global muscle strength (44), age-related declines in muscle strength are more pronounced in the lower extremities (45). Our study did not evaluate lower extremity muscle strength, which might have led to a failure in detecting differences in muscle strength between the ACTN3 genotypes. Additionally, the age range of our study population (middle-aged to older adults) could have influenced the results. Age-related atrophy of fast-twitch muscle fibers (46) may have obscured differences in SMM and strength between ACTN3 genotypes. These points highlight the need for methodological refinement in future studies, particularly with diverse age groups and more comprehensive strength assessments. Given these considerations, the results of our study should be interpreted cautiously, recognizing the inherent challenges and methodological constraints.
This study has several limitations. First, this study was cross-sectional; thus, causal relationships remain unclear. Second, coffee and green tea consumption was evaluated using FFQ and therefore was not classified based on the exact amount consumed. Differences in habitual means of drinking, such as cups, cans, and bottles, might have caused misclassification. Third, information on the use of sugar and milk in habitually consumed coffee was not collected and, therefore, was not analyzed. Fourth, the SMM estimated using the bioimpedance device might have had a prediction error compared with the SMM measured using the gold standard methods.
In conclusion, our study suggests a potential interaction between habitual green tea consumption and the ACTN3 genotype in association with HGS in females. In particular, within the ACTN3 XX genotype, habitual consumption of green tea was associated with higher HGS in females. However, the current data remain insufficient to elucidate the underlying mechanism associated with these observations; therefore, further studies are warranted to validate these findings and explore the potential mechanisms involved.
Acknowledgments: The authors thank Takumi Tokuda, Chiaki Shigetomi, Takaaki Komiyama, Hikaru Kawasaki, Yujiro Kose, Masaki Maehara, and Yuta Yoshitomi for their assistance with the second survey.
Funding: This study was supported by Grants-in-Aid for Scientific Research for Priority Areas of Cancer (No. 17015018), Innovative Areas (No. 221S0001), and the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers 18390182, 20249038, 16H06277, 20H03943, 16K09058, 21K11679, 22H04923 [CoBiA] and 22K21086) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. This research was also funded by the Hachiro Honjo Ocha Foundation.
Conflicts of interest: None declared.
Ethical standards: The research protocol was approved by the ethics committees of the Saga University Faculty of Medicine (approval no. 17-11) and Nagoya University Graduate School of Medicine (approval no. 253).
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. Oct 12 2019;394(10206):1365-1375. doi:10.1016/s0140-6736(19)31786-6
2. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. Jul 1 2019;48(4):601. doi:10.1093/ageing/afz046
3. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. Mar 2001;56(3):M146-56. doi:10.1093/gerona/56.3.m146
4. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res Rev. Jul 2017;36:1-10. doi:10.1016/j.arr.2017.01.006
5. Pratt J, Boreham C, Ennis S, Ryan AW, De Vito G. Genetic Associations with Aging Muscle: A Systematic Review. Cells. Dec 19 2019;9(1). doi:10.3390/cells9010012
6. Zempo H, Miyamoto-Mikami E, Kikuchi N, Fuku N, Miyachi M, Murakami H. Heritability estimates of muscle strength-related phenotypes: A systematic review and meta-analysis. Scand J Med Sci Sports. Dec 2017;27(12):1537-1546. doi:10.1111/sms.12804
7. Pickering C, Kiely J. ACTN3, Morbidity, and Healthy Aging. Front Genet. 2018;9:15. doi:10.3389/fgene.2018.00015
8. Nanri H, Yoshida T, Watanabe Y, et al. The Association between Habitual Green Tea Consumption and Comprehensive Frailty as Assessed by Kihon Checklist Indexes among an Older Japanese Population. Nutrients. Nov 19 2021;13(11). doi:10.3390/nu13114149
9. Huang L, Chen H, Liang M. The Association Between Habitual Tea Consumption and Frailty Transition in Community-Dwelling Older Adults: A Prospective Cohort Study. J Am Med Dir Assoc. Jul 13 2023. doi:10.1016/j.jamda.2023.06.006
10. Chua KY, Li H, Lim WS, Koh WP. Consumption of Coffee, Tea, and Caffeine at Midlife, and the Risk of Physical Frailty in Late Life. J Am Med Dir Assoc. Jul 21 2023. doi:10.1016/j.jamda.2023.06.015
11. Iwasaka C, Yamada Y, Nishida Y, et al. Association between habitual coffee consumption and skeletal muscle mass in middle-aged and older Japanese people. Geriatr Gerontol Int. Oct 2021;21(10):950-958. doi:10.1111/ggi.14264
12. Kawakami R, Tanisawa K, Ito T, et al. Coffee consumption and skeletal muscle mass: WASEDA’S Health Study. Br J Nutr. Jul 14 2023;130(1):127-136. doi:10.1017/s0007114522003099
13. Mazeaud S, Castellana F, Coelho-Junior HJ, et al. Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review. Metabolites. Jul 15 2022;12(7). doi:10.3390/metabo12070654
14. Li P, Liu A, Xiong W, et al. Catechins enhance skeletal muscle performance. Crit Rev Food Sci Nutr. 2020;60(3):515-528. doi:10.1080/10408398.2018.1549534
15. Kim H, Suzuki T, Saito K, et al. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. Geriatr Gerontol Int. Apr 2013;13(2):458-65. doi:10.1111/j.1447-0594.2012.00923.x
16. Dirks-Naylor AJ. The benefits of coffee on skeletal muscle. Life Sci. Dec 15 2015;143:182-6. doi:10.1016/j.lfs.2015.11.005
17. Hara M, Higaki Y, Imaizumi T, et al. Factors influencing participation rate in a baseline survey of a genetic cohort in Japan. J Epidemiol. 2010;20(1):40-5. doi:10.2188/jea.je20090062
18. Hara M, Shimanoe C, Otsuka Y, et al. Factors associated with non-participation in a face-to-face second survey conducted 5 years after the baseline survey. J Epidemiol. 2015;25(2):117-25. doi:10.2188/jea.JE20140116
19. Hamajima N, Group JMS. The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev. Apr-Jun 2007;8(2):317-23.
20. Iwasaka C, Yamada Y, Nishida Y, et al. Association of appendicular extracellular-to-intracellular water ratio with age, muscle strength, and physical activity in 8,018 community-dwelling middle-aged and older adults. Arch Gerontol Geriatr. Jan 18 2023;108:104931. doi:10.1016/j.archger.2023.104931
21. Iwasaka C, Yamada Y, Nishida Y, et al. Association Between the Appendicular Extracellular-to-Intracellular Water Ratio and All-Cause Mortality: A 10-Year Longitudinal Study. J Gerontol A Biol Sci Med Sci. Feb 1 2024;79(2). doi:10.1093/gerona/glad211
22. Hara M, Hachiya T, Sutoh Y, et al. Genomewide Association Study of Leisure-Time Exercise Behavior in Japanese Adults. Med Sci Sports Exerc. Dec 2018;50(12):2433-2441. doi:10.1249/mss.0000000000001712
23. Tokudome Y, Goto C, Imaeda N, et al. Relative validity of a short food frequency questionnaire for assessing nutrient intake versus three-day weighed diet records in middle-aged Japanese. J Epidemiol. Jul 2005;15(4):135-45. doi:10.2188/jea.15.135
24. Imaeda N, Goto C, Sasakabe T, et al. Reproducibility and validity of food group intake in a short food frequency questionnaire for the middle-aged Japanese population. Environ Health Prev Med. Mar 2 2021;26(1):28. doi:10.1186/s12199-021-00951-3
25. Nanri H, Hara M, Nishida Y, et al. Association between green tea and coffee consumption and body iron storage in Japanese men and women: a cross-sectional study from the J-MICC Study Saga. Front Nutr. 2023;10:1249702. doi:10.3389/fnut.2023.1249702
26. Yamada Y, Schoeller DA, Nakamura E, Morimoto T, Kimura M, Oda S. Extracellular water may mask actual muscle atrophy during aging. J Gerontol A Biol Sci Med Sci. May 2010;65(5):510-6. doi:10.1093/gerona/glq001
27. Kaysen GA, Zhu F, Sarkar S, et al. Estimation of total-body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy. Am J Clin Nutr. Nov 2005;82(5):988-95. doi:10.1093/ajcn/82.5.988
28. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. Feb 15 2004;159(4):413-21. doi:10.1093/aje/kwh058
29. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. Mar 2020;21(3):300-307 e2. doi:10.1016/j.jamda.2019.12.012
30. Hishida A, Nakatochi M, Akiyama M, et al. Genome-Wide Association Study of Renal Function Traits: Results from the Japan Multi-Institutional Collaborative Cohort Study. Am J Nephrol. 2018;47(5):304-316. doi:10.1159/000488946
31. North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet. Apr 1999;21(4):353-4. doi:10.1038/7675
32. Yang N, MacArthur DG, Gulbin JP, et al. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet. Sep 2003;73(3):627-31. doi:10.1086/377590
33. Nishida Y, Higaki Y, Taguchi N, et al. Objectively measured physical activity and inflammatory cytokine levels in middle-aged Japanese people. Prev Med. Jul 2014;64:81-7. doi:10.1016/j.ypmed.2014.04.004
34. Kiyama R. Estrogenic biological activity and underlying molecular mechanisms of green tea constituents. Trends in Food Science & Technology. 2020/01/01/ 2020;95:247-260. doi: 10.1016/j.tifs.2019.11.014
35. Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone Therapy and Skeletal Muscle Strength: A Meta-Analysis. The Journals of Gerontology: Series A. 2009;64A(10):1071-1081. doi:10.1093/gerona/glp082
36. Collins BC, Laakkonen EK, Lowe DA. Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength. Bone. 2019/06/01/ 2019;123:137-144. doi: 10.1016/j.bone.2019.03.033
37. Del Coso J, Hiam D, Houweling P, Pérez LM, Eynon N, Lucía A. More than a ‘speed gene’: ACTN3 R577X genotype, trainability, muscle damage, and the risk for injuries. Eur J Appl Physiol. Jan 2019;119(1):49-60. doi:10.1007/s00421-018-4010-0
38. Holloszy JO, Faulkner JA, Brooks SV, Zerba E. Muscle Atrophy and Weakness With Aging: Contraction-Induced Injury as an Underlying Mechanism. The Journals of Gerontology: Series A. 1995;50A(Special_Issue):124-129. doi:10.1093/gerona/50A.Special_Issue.124
39. Lee DY, Shin S. Sarcopenic obesity is associated with coffee intake in elderly Koreans. Front Public Health. 2023;11:990029. doi:10.3389/fpubh.2023.990029
40. Grgic J, Grgic I, Pickering C, Schoenfeld BJ, Bishop DJ, Pedisic Z. Wake up and smell the coffee: caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses. Br J Sports Med. Jun 2020;54(11):681-688. doi:10.1136/bjsports-2018-100278
41. Ommati MM, Farshad O, Mousavi K, Khalili M, Jamshidzadeh A, Heidari R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia. 2020;75:1221-1230. doi:10.2478/s11756-020-00429-7
42. Tsuda S, Egawa T, Ma X, Oshima R, Kurogi E, Hayashi T. Coffee polyphenol caffeic acid but not chlorogenic acid increases 5’ AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. The Journal of nutritional biochemistry. 2012;23(11):1403-1409. doi: 10.1016/j.jnutbio.2011.09.001
43. Buckinx F, Landi F, Cesari M, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. Apr 2018;9(2):269-278. doi:10.1002/jcsm.12268
44. Porto JM, Nakaishi APM, Cangussu-Oliveira LM, Freire Júnior RC, Spilla SB, Abreu DCC. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr. May-Jun 2019;82:273-278. doi:10.1016/j.archger.2019.03.005
45. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. May 2001;56(5):B209-17. doi:10.1093/gerona/56.5.b209
46. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. Nov 1995;50 Spec No:11-6. doi:10.1093/gerona/50a.special_issue.11
The Author(s) 20224