T. Byrne1, J. Cooke2, E. McNeela1, P. Bambrick3, R.P. Murphy4, M. Harrison5
1. Pharmaceutical, Molecular and Biotechnology Research Centre and Department of Science, South East Technological University, Waterford, Ireland; 2. Department of Geriatric Medicine, University Hospital Waterford, Waterford, Ireland and Department of Medicine, Royal College of Surgeons in Ireland, Dublin, Ireland; 3. Department of Geriatric Medicine, University Hospital Waterford, Waterford, Ireland; 4. School of Health and Human Performance, Dublin City University, Dublin 9, Ireland; 5. Pharmaceutical, Molecular and Biotechnology Research Centre and Department of Sport and Exercise Science, South East Technological University, Waterford, Ireland.
Corresponding Author: Michael Harrison, South East Technological University – Waterford Campus: South East Technological University, Ireland, michael.harrison@setu.ie
J Frailty Aging 2024;13(3)203-212
Published online May 7, 2024, http://dx.doi.org/10.14283/jfa.2024.38
Abstract
BACKGROUND: There is a need to identify vascular and geroscience-relevant markers and mediators that can physiologically link ageing to vascular disease. There is evidence of specific T cell subsets, all influenced by age, that exert positive and negative effects on vascular health. CD31+, termed angiogenic T cells, have been linked to vascular repair whereas CD28null, termed senescent T cells, display pro-inflammatory and cytotoxic effector functions.
OBJECTIVE: This study sought to determine the combined influence of increasing age and frailty status on these circulating CD31+ and CD28null T cell subsets.
METHODS: This cross-sectional study recruited four different cohorts of men and women; young (20-30 years, n=22), older (65-75 years, n=17), robust non-frail (76+ years, n=17), and frail (76+ years, n=15) adults. Frailty was determined using the Fried Frailty method. T cell subsets were determined by whole blood flow cytometry based on the expression of CD3, CD4, CD8, CD31 and CD28. Cognitive impairment (CI) was measured via the Montreal Cognitive Assessment test.
RESULTS: Whether expressed as circulating counts or as a % of total T cells, there was a progressive decrease (p<0.05) in CD31+ T cells with increasing age but paradoxically higher values (p<0.05) in the frail compared to the robust non-frail group. These changes were similar in the CD4+ and CD8+ fractions. CD28null T cells were considerably higher (p<0.05) in the frail compared to the robust non-frail group, including in the CD8+ (47% vs 29%, p<0.05) and CD4+ (4% vs 1%, p<0.05) fractions. CD28null T cell percentage was also higher (p<0.05) in those with moderate CI compared to mild CI and normal function.
CONCLUSION: CD8+CD28null T cells are considerably elevated in frailty and with cognitive impairment and may serve as a useful target for intervention. Currently, the utility of CD31+ T cells as an ageing biomarker may be confined to healthy ageing cohorts.
Key words: T cell, biomarker, inflammaging, geroscience, frail, age.
Introduction
Frailty is a medical syndrome most often observed in older adults that results from a simultaneous decline in multiple physiological systems leading to a decline in the overall physiological function of the individual. This multisystem decline leaves individuals at increased risk of adverse events such as acute illness, falls, institutionalisation, hospitalisation, and mortality (1, 2). Age-related frailty is an increasing challenge for societies worldwide, with a growing emphasis on identifying its underlying pathophysiology and prospects for intervention (3).
Although not explicitly part of the syndrome based on some definitions employed, there is an important cardiovascular dimension to frailty. The prevalence of frailty is higher in populations with cardiovascular disease (4) and frail persons without clinical cardiovascular disease at baseline have been demonstrated to be at increased risk of future cardiovascular events (5). The observed associations between frailty and vascular disease are complex, and it is not currently clear whether a frailty diagnosis increases the risk of future vascular disease or whether having a vascular condition increases the risk of developing frailty. Epidemiological evidence supports both scenarios (5, 6). Mechanistic links between frailty and vascular cognitive impairment have been proposed (7). In addition, pulse wave velocity, a physiological indicator of arterial stiffness linked to vascular risk and cognitive decline (8) is increased with age (9) and with frailty (10).
Multiple changes occur in the components of the immune system with age and frailty, many of which have previously been extensively reviewed (11, 12). The effects of thymic involution are particularly evident on the T lymphocyte component of the adaptive immune system with these changes frequently studied in older individuals in the context of immune function and response to vaccination (13). Less recognised are the implications of age-related T cell changes for processes related to vascular injury and repair. T cell subsets have been positively and negatively implicated in vascular health and age-related vascular disease.
A T cell population has been identified expressing platelet endothelial cell adhesion molecule (PECAM or CD31), that promotes endothelial repair and revascularisation. Hur and colleagues (14) described these cells as «angiogenic T cells». Unlike CD31 negative T cells, these CD31+ T cells restored muscle blood flow when injected into T cell deficient mice with hindlimb ischemia. They demonstrated superior angiogenic potential concerning tube formation, adhesion and transendothelial migration, and secretion of angiogenic cytokines (14). CD3+CD31+ T cells have also been positively associated with endothelial function and negatively associated with several cardiovascular disease risk factors (14, 15). In humans, it has been demonstrated that circulating counts and percentage frequency of angiogenic T cells decrease with age (16, 17).
In contrast, senescent T cells represent a T cell population towards the end of the naïve – terminally differentiated continuum, that exhibit increased inflammatory cytokine production and cytotoxic effector functions, but resistance to apoptosis (18). They no longer express the co-stimulatory molecule CD28 and this surface marker characteristic is frequently used to identify them in the circulation using flow cytometry. Other signature markers expressed include CD57 and killer cell lectin-like receptor G1. Senescent T cells potentially trigger inflammation in several inflammatory disorders, including atherosclerosis (19). Due in part to thymic involution and lifelong exposure to chronic infections, senescent T cells are linked to the immunosenescence of advancing age (20). This expansion in CD28null T cells with age is particularly evident in the CD8+ cytotoxic T cell fraction (21). The relationship with age-related frailty is less frequently considered though associations of senescent T cell subsets and incident frailty have previously been reported (22, 23). More recently, there has been interest in a hybrid cell phenotype, the CD28null CD31+ T cell identified by Lopez and colleagues (24) though these cells appear to also display a cytotoxic inflammatory profile (24).
Geroscience explores biological mechanisms of ageing as targets for intervention that may delay the physiological consequences of ageing, maintain function, and prevent frailty and disability (25). Cellular senescence has been identified as one of the geroscience hallmarks of ageing (26). These angiogenic and senescent T cell subsets merit further investigation as biomarkers that relate to the hallmarks of ageing and to vascular disease. There is biological plausibility to an influence as impaired vascular repair, cellular senescence and inflammation are key features of ageing and the athero-arteriosclerotic processes. There is a clear need to identify age- and frailty-related vascular phenotypes in older populations that will further the understanding of underlying pathophysiologies and ultimately form the basis for monitoring intervention efficacy. Though limited evidence exists of an age or frailty effect on these immuno-vascular markers, to our knowledge, these angiogenic and senescent T cells and their subsets have not been studied together within a single study design. The purpose of this cross-sectional study was therefore to investigate the combined effect of age and frailty on circulating angiogenic and senescent T cell populations.
Methods
Study design
This study was a cross-sectional design comparing circulating T cell and other immune subsets in a young adult (20-30 yrs) and three older adult groups, older (65-75 yrs), robust non-frail (76+ yrs) and frail (76+ yrs). Descriptive characteristics of the four study cohorts are displayed in table 1. The effect of age was determined with comparisons between the young, older and robust non-frail 76+ groups. The effect of frailty was determined by comparing the robust non-frail 76+ and frail 76+ groups.
Recruitment, Exclusion, Ethics, and Consent
All non-frail participants were recruited in community settings. Frail participants were recruited from the University Hospital Waterford (UHW) Department of Medicine for the Elderly clinics and local residential care facilities. Exclusion criteria for any of the four groups included physical or cognitive limitations rendering the participant unable to provide informed consent or complete the Fried Frailty Index assessment, known inflammatory or autoimmune disorders, active malignancy, vascular events in the previous 6 months or on prescribed medications known to influence immune cell populations (e.g. corticosteroids, monoclonal antibodies or immunotherapeutics). Informed consent was obtained for all participants in the study. Ethical approval for the study was obtained from the Waterford Institute of Technology (now South East Technological University) Research Ethics Committee and the Research Ethics Committee, Health Services Executive, South East.
Preparation for assessments
In the 48 hours before blood collection, all participants were asked to refrain from vigorous physical activity and alcohol consumption. On the morning of blood collection, all participants were advised to avoid caffeine and consume only a light breakfast consisting of no more than two slices of toast, a piece of fruit, or a bowl of cereal.
Frailty assessment
Frailty status was determined using a modified version of the Fried Frailty Index (1) with reference to its five criteria: unintentional weight loss, self-reported exhaustion, low gait speed, reduced grip strength and low physical activity. Low physical activity was defined by a negative response to the question «Do you accumulate more than 30 minutes walking on most days of the week?» AND a positive response to the question «Do you sit for most of the day?» Pre-frail older adults (1 or 2 criteria) were not included in the study with robust (0 criteria) and frail (3 – 5 criteria) participants eligible for the non-frail 76+ and frail 76+ groups respectively.
The Montreal Cognitive Assessment (MoCA) is a screening tool to measure cognitive function (27). The MoCA identifies eight domains that are most seen to decline: executive functioning, naming, memory, attention, language, abstraction, delayed recall, and orientation. It was administered using a standardised protocol. Based on MoCA scores, participants were classified as having normal cognitive function (26-30 points), mild cognitive impairment (18-25 points) or moderate cognitive impairment (10-17 points).
Physical activity was determined via a suitable version of the International Physical Activity Questionnaire (IPAQ), a validated tool (28). Young adults completed the standard IPAQ short form, and older adults completed the IPAQ-Elderly (IPAQ-E) short form. The IPAQ-E has been validated for use in populations over 65 years old (29).
Physiological measurements
The anthromopetric assessments undertaken were height, weight and waist circumference with body mass index derived. Body fat percentage was determined by multi-frequency bioelectrical impedance (Bodystat Quadscan 4000 BIA, Bodystat Ltd., Isle of Man, British Isles). Blood pressure was measured using an automated sphygmomanometer (Omron M2, Omron Healthcare, UK) following 5 minutes of seated rest. Three separate measurements were taken and the average recorded. Carotid-femoral pulse wave velocity, a measure of aortic stiffness was determined using the Complior Analyse instrument (ALAM Medical, France) following 5 min of supine rest. The distance between the carotid and femoral pulse sensors was determined by tape measure as the shortest direct distance.
Blood sampling
Blood was sampled from a forearm vein by 21-gauge venupuncture. Blood tubes were kept at room temperature and processed within 2 hours of collection. The first 1 mL of blood was collected into a discard tube. Two tubes were prepared for lysed whole blood flow cytometric analysis, one for a T cell assay and one for a monocyte assay. A full blood count was also obtained using an automated haematology analyser (Ac·T diff2, Beckman Coulter, USA).
Flow cytometry
Separate T cell and monocyte assays were undertaken using multicolour flow cytometry (Cytomics FC500, Beckman Coulter, USA). Data analysis (percentage expression and counts/µL) was carried out using CXP software version 2.3. Fluorescence compensation parameters were established using single stained compensation beads and the cytometer flow rate was monitored by adding AccuCount beads (Spherotech Inc, Illinois, USA) to samples.
T cell and monocytes assay overview
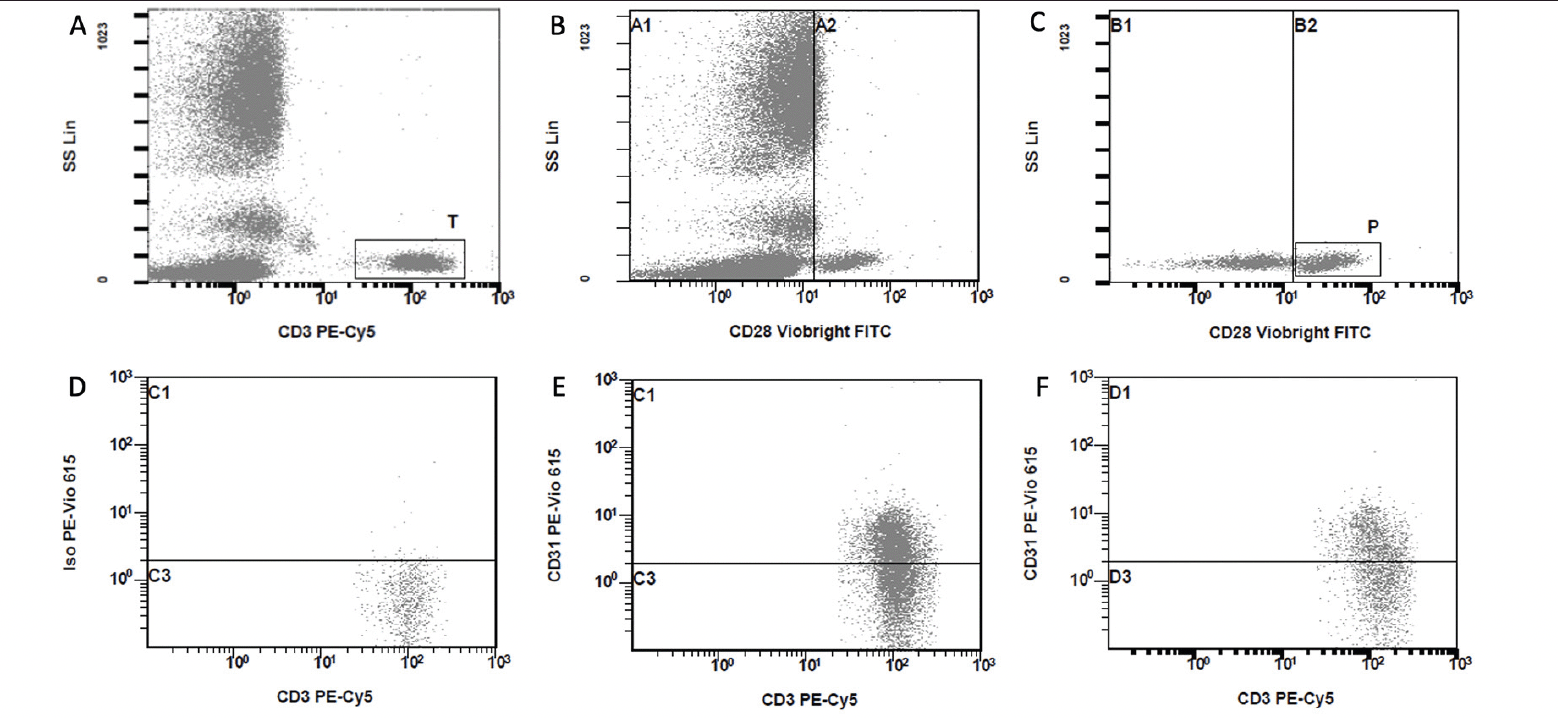
The T cell assay tube contained anti-CD3, CD4, CD8, CD31 and CD28 antibodies. Angiogenic T cells were defined as CD3+CD31+. Senescent T cells were defined as CD3+CD28null. We also enumerated CD3+CD31+CD28+ T cells to distinguish from CD3+CD31+CD28null T cells which are assumed to be cytotoxic. All subsets can be further broken down into the CD3+CD4+ and CD3+CD8+ T cell fractions. A representative profile of the flow cytometry gating strategy for the T cell assay is presented in figure 1. The monocyte assay tube contained anti-CD14, CD16 and Tie2 antibodies with CD14 and CD16 expression used to identify classical, intermediate and non-classical monocyte fractions based on the method of Weber and colleagues (30). This monocyte gating strategy used in our lab has been previously presented (31).
T cell gate [T] based on CD3 Vs SS (A). CD28+ and CD28null lymphocyte threshold identified visually from CD28 vs SS plot (B). CD28+ and CD28null T cells gated on [T] with threshold from (B), CD28+ events gated as [P] (C). Negative control sample to establish threshold for CD31+ and CD31- populations gated on T (D). CD3+CD31+ T cells from CD3 vs CD31 plot gated on [T] with threshold from D (E). CD3+CD31+CD28+ T cells from CD3 vs CD31 plot gated on [T] and [P] with threshold from D (F). Similar approach taken with identification of CD4+ and CD8+ T cell subsets with gating initially on [T] followed by gating on a CD4 vs SS or CD8 vs SS plots followed by steps C – F. Representative plots shown here were generated using blood obtained from a frail participant.
Staining and analysis protocol
The fluorochrome-conjugated monoclonal antibodies used in both assays were all, unless otherwise stated, REAfinity recombinant antibodies (Miltenyi Biotec, UK) which are IgG1 isotype class. The following antibodies were used to differentiate various T cell subsets; anti-CD3-PE-Cy5, IgG2a,κ (BD Biosciences, UK), anti-CD4-PE REA623, anti-CD8-PE-Vio770 REA734, anti-CD31-PE-Vio615 REA1028, anti-CD28 Viobright FITC REA612 and REAControl (S)-PE-Vio615 REA293. The following antibodies were used to differentiate monocyte subsets; anti-CD14-PE REA599, anti-CD16-PEVio615 REA423, anti-CD202b (Tie2) PE-Vio770 REA198 and REA Control (S)-PE-Vio770 REA 293.
Briefly, 100 µL of EDTA whole blood was added to a 5 mL flow cytometry tube followed by a fixed volume of antibody, determined by titration. For the 5-colour T cells assay, one sample tube and one negative control tube (4 colours and PE-Vio615 isotype control) were required per participant to distinguish between CD31+ and CD31- events. For the 3-colour monocyte assay, one sample tube and one isotype control tube (2 colours and PE-Vio 770 isotype control) were required per participant to distinguish between Tie2+ and Tie2- events. Tubes were incubated in the dark for 20 minutes at room temperature. Next, 1 mL of 1:10 diluted lysis buffer (NH4Cl – 1.5M, NaHCO3 – 100mM, EDTA – 10mM, pH 7.4) was added with a further incubation for 15 min. Finally, 50 µL of the flow count beads were added to the sample tube immediately prior to cytometer analysis at medium speed. The T cell and monocyte tubes were run for 300 s and 900 s respectively to yield sufficient events including rare subsets.
Statistical analysis
All data were analysed using SPSS. Normality was checked for all variables using the Shapiro-Wilk test. With one young group, two essentially healthy older adult groups and one frail group, the data was invariably skewed. Non-normal variables were natural log-transformed prior to inferential statistical analysis. Values across the four groups were compared using a one-way Analysis of Covariance (ANCOVA), with sex included as a covariate in all analyses. The key comparisons of interest were between (a) the young, older and non-frail 76+ groups as these were relevant to an age effect and (b) between non-frail 76+ and frail 76+ as these were relevant to a frailty effect. Separately, values across those with normal cognitive function, mild cognitive impairment and moderate cognitive impairment (drawn from the older, non-frail 76+ and frail 76+ groups) were compared using a one-way Analysis of Covariance (ANCOVA), with age and sex included as a covariate in all analyses. The key outcome measures were also compared between men and women using a one-way ANCOVA with age as the covariate. If a significant main effect was detected in these ANCOVA analyses, pairwise comparisons were undertaken using the Least Significant Difference test. Significance was set at p < 0.05. In the case of log transformed variables, summary data (mean, 95% confidence interval) for each group were then back-transformed to give more meaningful results for presentation in graphs and tables. Unless stated, all variables are presented as mean (95% confidence interval) based on initial or back-transformed values. Correlation analysis between key angiogenic and senescent cell subsets of interest and vascular outcomes were carried out using Pearson’s partial correlation, controlling for age and sex.
Results
With the exception of CD31 positivity on CD8+ T cells (mean ± SEM; men 61 ± 2%, women 74 ± 2%, p<0.05), there was no effect (p>0.05) of sex on all other whole blood, angiogenic T cell and senescent T cell subsets including in the CD4+ and CD8+ fractions (data not shown).
Body composition, vascular, and functional outcome measures
A range of descriptive, body composition, vascular, cognitive, and functional characteristics of participants are shown in table 1. There was a small age difference between the non-frail 76+ and frail 76+ groups (despite efforts to age match). Physical activity, cognitive function, 15-foot walk time (FWT) and grip strength were all lower (p<0.05) in the frail 76+ compared to the non-frail 76+ group. In general, systolic blood pressure, diastolic blood pressure, and pulse wave velocity were not different (p>0.05) across the older, non-frail 76+ and frail 76+ groups but lower (p < 0.05) in the young group.
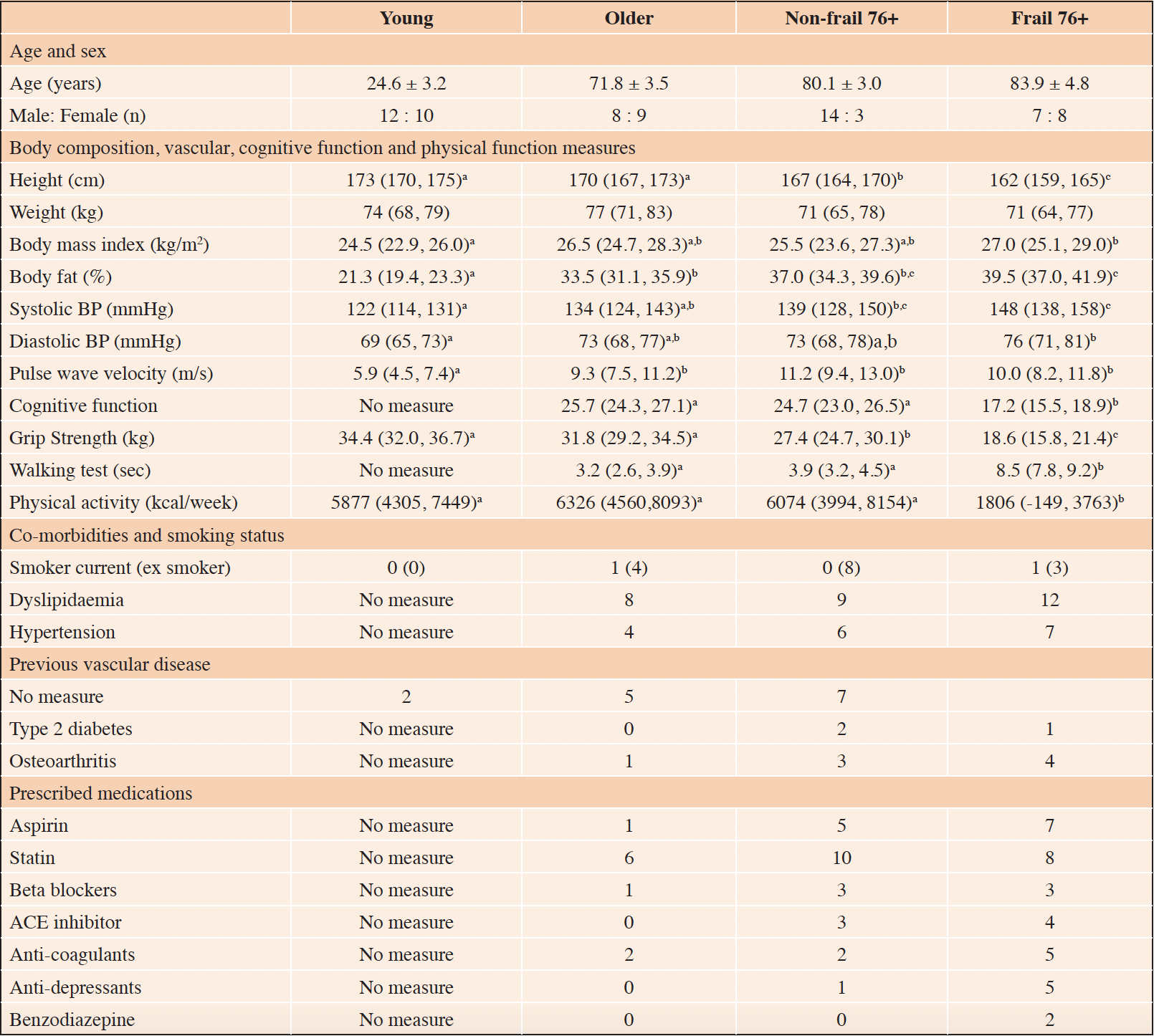
Table 1. Descriptive characteristics of the young (n = 22), older (n = 17), non-frail 76+ (n=17) and frail 76+ (n = 15) study participants
Sex, co-morbidities and prescribed medications as n=. Age as mean ± SD. Body composition, vascular, cognitive function and physical function measures data presented as mean (95% confidence interval). Datapoint letters used to indicate significant differences (p < 0.05). Datapoints which share letters are not significantly different. Analysis based on one-way ANCOVA with sex as covariate followed up with Least Significant Difference pairwise comparisons. No letters indicate no significant main effect of group. 15-FWT = 15 foot walk test. BP = blood pressure.
Haematology outcome measures
The whole blood haematology and T cell count data are shown in Table 2 and in Figure 2. There was an increase in total leukocytes, granulocytes and monocytes with age, with significant differences (p<0.05) between the young and non-frail 76+ groups. There were increases in the granulocyte to lymphocyte ratio and the monocyte to lymphocyte ratio with age (more granulocytes and monocytes per lymphocyte), with significant differences (p<0.05) between the young and non-frail 76+ groups. These haematology values were not significantly different (p>0.05) between non-frail 76+ and frail 76+ groups. CD3+ T cells decreased with age with a significant difference ((p<0.05) between the young and non-frail 76+ groups (figure 2). There was no significant main effect for group for CD4+ T cells (Table 2). There was a progressive decrease (p<0.05) in CD8+ T cells with age (Table 2). CD8+ T cell counts were higher in the frail 76+ compared to the non-frail 76+ groups (Table 2).
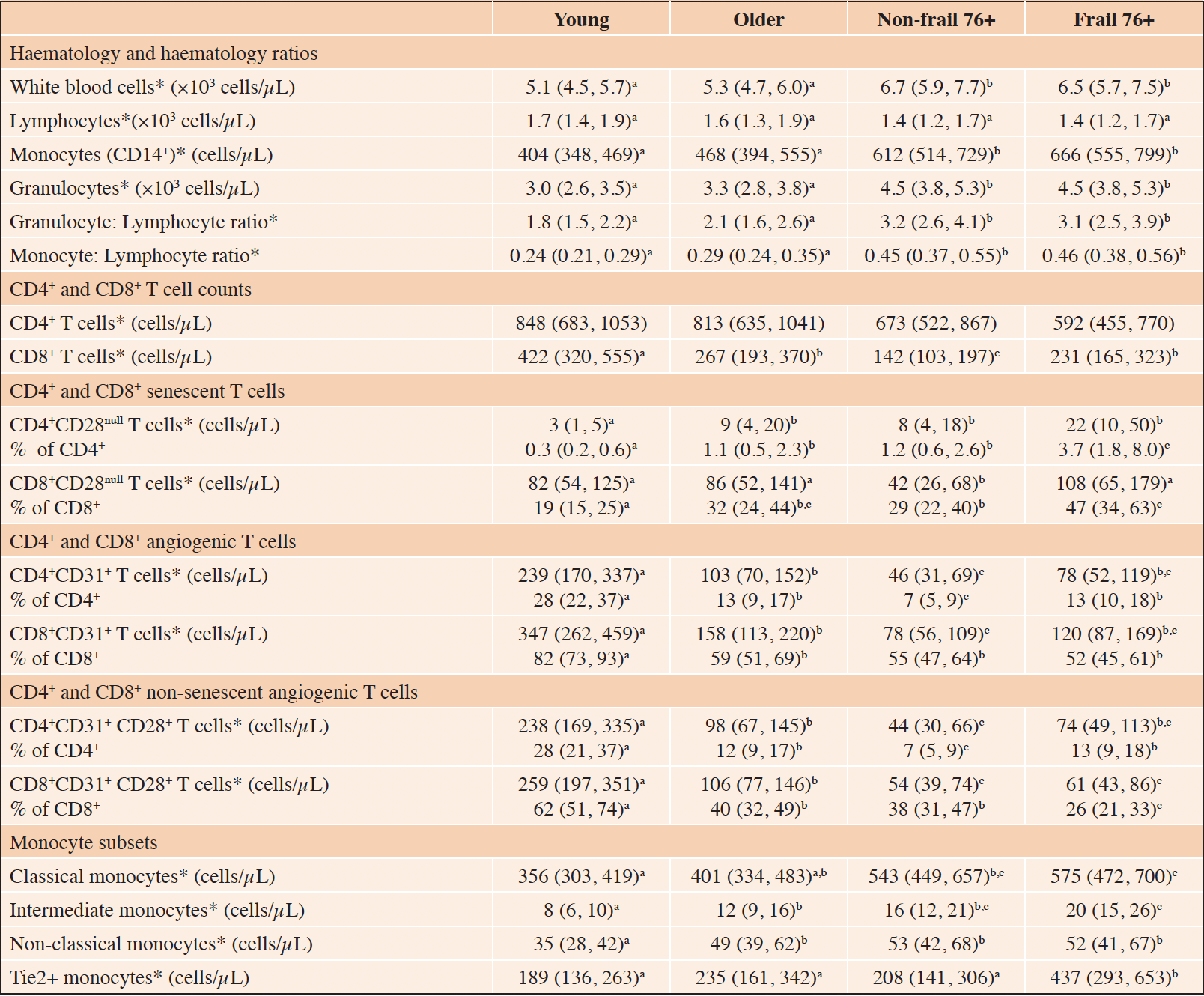
Table 2. Complete blood count and selected T cell characteristics of the young 20-30 (n = 22), older (n = 17), non-frail (n=17) and frail (n = 15) study participants
Data presented as mean (95% confidence interval). Datapoint letters used to indicate significant differences (p < 0.05). Datapoints which share letters are not significantly different. Analysis based on one-way ANCOVA with sex as covariate followed up with Least Significant Difference pairwise comparisons. No letters indicate no significant main effect of group. * Analysis for this variable based on natural log-transformed data with tabulated mean and confidence intervals presented as back-transformed data.
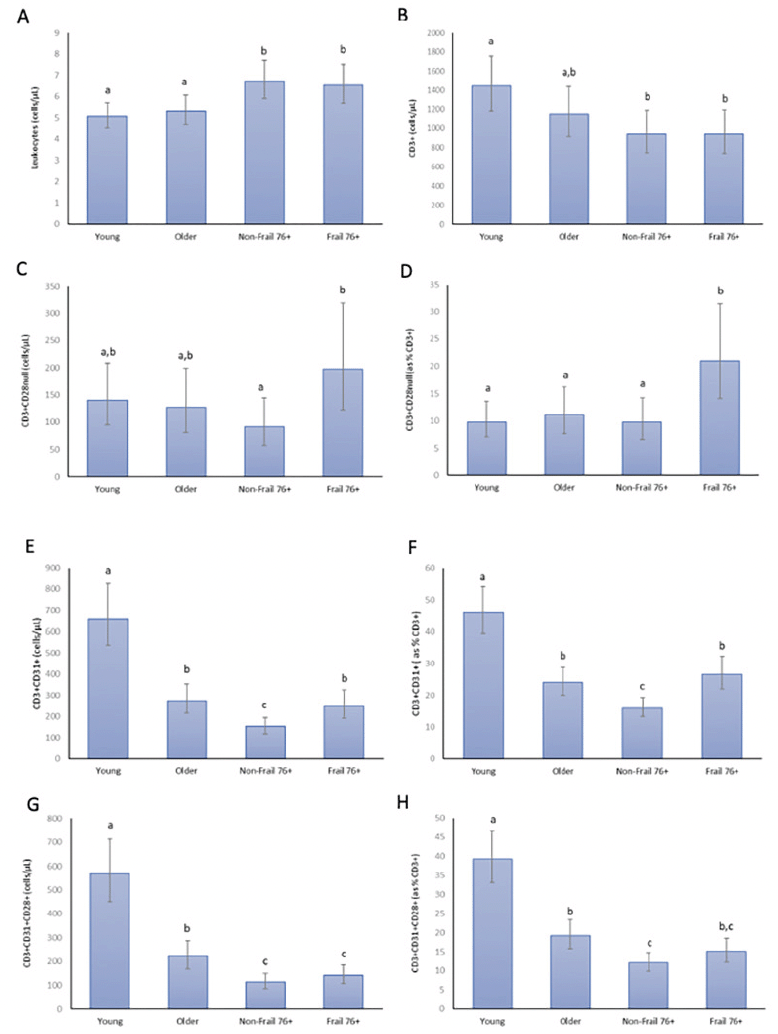
Figure 2. Leukocyte counts (A), CD3+ T cells (B), CD3+CD28nullcells (C), CD28null T cell percentages (D), CD3+CD31+ T cells (E), CD31+ T cell percentages (F), CD3+CD31+CD28+ T cells (G) and CD31+CD28+ T cell percentages (H) in young (n=22), older (n=17), non-frail 76+ (n=17) and frail 76+ (n=15) participants
All analyses based on natural log-transformed data with figure means and 95% confidence interval error bars presented as back-transformed data. Datapoint letters used to indicate significant differences (p < 0.05). Datapoints which share letters are not significantly different.
CD28null senescent T cells
The proportion of CD4+ and CD8+ T cells that were CD28null was higher (p<0.05) in the older and non-frail 76+ groups compared to the young group (Table 2) indicating a small age effect. There was also a small age effect (p<0.05) for CD4+CD28null and CD8+CD28null T cell counts (Table 2). There was no significant age effect however for CD3+CD28null T cell counts or percentages (Figure 2 C & D). CD3+CD28null and CD8+CD28null counts and percentages and CD4+CD28null percentages were all higher (p<0.05) in the frail 76+ compared to the non-frail 76+ groups (Figure 2, Table 2). CD28 negativity was considerably greater in the CD8+ compared to the CD4+ T cell fraction (47% vs 4% for frail participants, p<0.05).
Senescent T cells were higher in participants with lower cognitive function. CD3+CD28null, CD3+CD4+CD28null and CD3+CD8+CD28null cell counts were all higher (p<0.05) in those with moderate cognitive impairment compared to those with mild impairment and normal cognitive function (Figure 3). The changes in T cell CD28 negativity across the cognitive function categories (Figure 3) did not reach significance (main effect > 0.05).
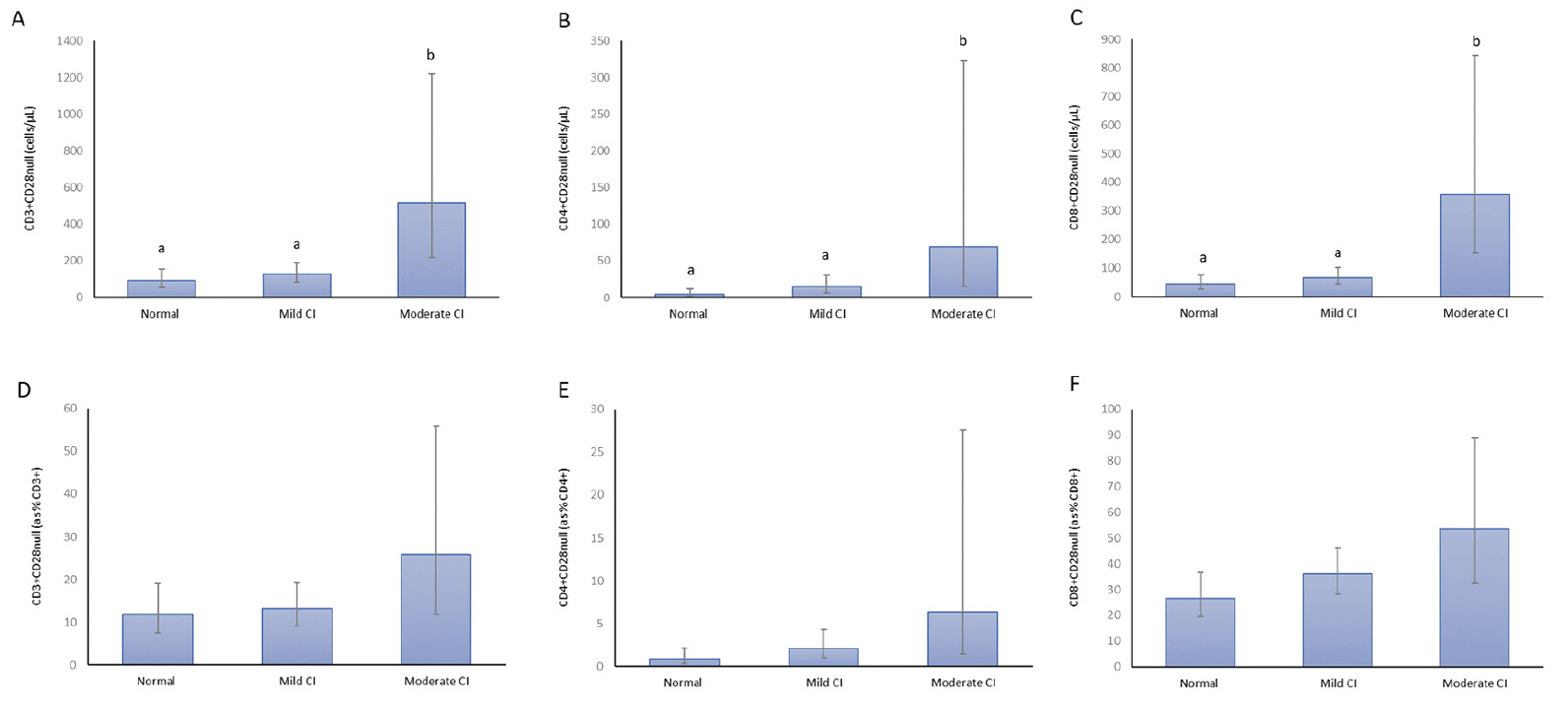
Figure 3. CD3+CD28null cells (A), CD3+CD4+CD28null cells (B) CD3+CD8+CD28null cells (C), CD28null CD3+T cell percentages (D), CD28null CD4+T cell percentages (E) and CD28null CD8+T cell percentages (F) in individuals over 65 years with normal cognitive function (n=16), mild cognitive impairment (n=22) and moderate cognitive impairment (n=7)
All analyses based on natural log-transformed data with figure means and 95% confidence interval error bars presented as back-transformed data. Datapoint letters used to indicate significant differences (p < 0.05). Datapoints which share letters are not significantly different.
CD3+CD31+ angiogenic T cells
Whether presented as cell counts or as a percentage of CD3+ events (Figure 2 E & F), an age effect was evident for CD3+CD31+ T cells. The older group had significantly lower (p<0.05) values compared to the young group, and the non-frail 76+ group had significantly lower (p<0.05) values again compared to the older group. The frail group had significantly higher (p<0.05) values compared to the non-frail 76+ group, reversing the downward trend with age. CD31 positivity was higher (p<0.05) in the CD8+ compared to the CD4+ T cell fraction (Table 2). When values are examined in the CD4+ and CD8+ fractions, similar age and frailty effects are evident for CD4+ and CD8+ T cell counts and for CD4+ percentage positivity (Table 2). Pulse wave velocity was inversely related to CD3+CD31+ positivity after adjusting for age and sex (r= – 0.27, p<0.05).
The proportion of CD31+ T cells not expressing CD28 (i.e. senescent) was 13%, 15%, 14% and 34% in the young, older, non-frail 76+ and frail 76+ groups respectively (p<0.05 when frail 76+ are compared to other groups, data not presented in graphs or tables). When these CD28null T cells are excluded from the analysis, there remained a similar age effect (p<0.05) on CD3+CD31+CD28+ T cells, both for cell counts and percentage positivity (Figure 2 G & H). There was no longer a significant increase due to frailty however in CD3+CD31+CD28+ or CD4+CD31+CD28+ T cell counts or in percentage positivity (Figure 2 G & H, Table 2). In the CD8+ fraction CD31+CD28+ T cell percentage positivity was lower (p<0.05) in frail 76+ compared to the non-frail 76+ groups (Table 2).
Monocytes
There was an age effect evident for total monocytes and for the classical, intermediate and non-classical monocyte fractions with increasing values (p < 0.05 between young and at least one of the other groups) across the age categories (Table 2). There was no significant effect (p>0.05) of frailty on total monocytes or on the classical, intermediate and non-classical fractions. The proportions of monocytes in the classical (circa 88%), intermediate (circa 3%) and non-classical (circa 9%) fractions were similar across the age and frailty groups. There was no age effect on Tie2 monocytes (p>0.05) but values were higher (p<0.05) in the frail 76+ compared to the non-frail 76+ groups (Table 2).
Discussion
Targeting the biology of ageing with appropriate interventions represents a novel way to extending human health including vascular health. This study sought to determine the effects of age and frailty on angiogenic and senescent T cells, immunovascular cells with geroscience biomarker potential. The aim was to identify a T cell phenotype with implications for the vascular dimensions of ageing and frailty that could be monitored in therapeutic interventions. The key findings were a progressive reduction in angiogenic CD31+ T cells with increasing age in non-frail individuals but a paradoxical increase with frailty. Senescent CD28null T cells were particularly prevalent in the CD8+ fraction. These were increased considerably in frailty, with only limited evidence of any progressive age effect.
Although previous studies (16, 17) have demonstrated a decrease in angiogenic T cells in older compared to younger individuals, we have extended the potential utility of these cells in ageing research by demonstrating differences in cell number and percentage between two older cohorts. The age effect on these CD31+ T cells was similar in the CD8+ and CD4+ T cell fractions, though CD31 expression is greater in the CD8+ fraction. The data suggest that the age effect on angiogenic T cell counts is primarily due to a reduction in T cell CD31 expression but also in part due to decreases in total CD3+ counts. The loss of these reparative cells may contribute to the increased vascular risk and increased incidence of vascular diseases observed in older adults.
The biological mechanism underlying the reduction in CD31 expression on T cells with increasing age is unclear. Repeated T cell activation over a lifetime, thymic involution and age-related oxidative stress are potential contributors. CD31 expression, at least on CD4 T cells, is considered from an immunological perspective to be a marker for recent thymic emigrants (32, 33) which will reduce with increasing age in the context of thymic involution. One study has shown that antibody-T cell receptor engagement and subsequent T cell activation can result in the loss of CD31 expression on the surface of T cells (34). Another study demonstrated that repeated stimulation and activation of neonatal T cells highly positive for CD31 (85 – 90%) resulted in the progressive loss of CD31 surface expression with each round of stimulation (35). Age-related oxidative stress which preferentially targets CD31+ T cells may also be influencing CD31+ and CD31- T cell proportions. CD31+ T cells have been reported as having functional characteristics that may render them more susceptible to apoptosis than their CD31- counterparts (16, 36). Oxidative stress is an established part of the ageing process (37) and has also been implicated in inducing apoptosis in naïve T cells (38).
An unexpected finding of this study was the paradoxical higher angiogenic T cells values with frailty. This is the first study to demonstrate higher cell counts and CD31+ proportions when over 76-year-old frail and robust non-frail groups are compared. The CD4+ and CD8+ T cell fractions appear to be equally responsible for higher frail 76+ group values. It is not clear however from this cross-sectional design if there is an increase in angiogenic T cells at the individual level around the onset of frailty or if these those on a frailty trajectory have had higher values than age-matched counterparts for many of the preceding years. If the former is the case, the reversal of a downward trend with age could mark progression to dysfunctional ageing. Longitudinal tracking studies would assist here.
Our study is not the first to observe an increase in CD31+ T cells in a pathological disease state. CD31+ T cell counts are higher in patients with various auto-immune conditions with related vascular pathologies including Sjӧgren’s Syndrome, systemic sclerosis, lupus and vasculitis (39-43). These observations are interesting in the context of a recent opinion identifying auto-immunity as an under-recognised contributor to sarcopenia (44) but also reviews highlighting the overlap between frailty and immune-mediated rheumatic diseases (45). Though no compelling evidence exists, it is suggested that such increases in specific disease states reflects increasing vascular damage and a need to stimulate endothelial progenitor cell function (41, 43). It has further been suggested that despite increases in CD31+ T cell counts in the circulation, CD31+ T cell function may be impaired in the pro-inflammatory environment of Sjӧgren’s Syndrome (39), with an implication that all CD31+ T cells are not similarly protective.
This paradoxical reduction with ageing but increase in frailty limits the value of CD31+ T cells to serve as target biomarkers in interventions that include participants likely to be in the frail range, unless the cell population of interest can be further characterised. Similar to the approach of Lopez and colleagues (24), we sought, with limited success, to refine the angiogenic phenotype by examining changes in non-senescent CD31+ T cells only. CD3+CD31+CD28+ and CD8+CD31+CD28+ T cells were no longer statistically higher in the frail 76+ group when we only enumerated CD31+ CD28+ events. Indeed, the CD31+CD28+ percentage positivity in the CD8+ T cell fraction was lower in the frail 76+ compared to the non-frail 76+ group. This is perhaps unsurprising given the proportion of CD8+ T cells that are CD28null. Although this preliminary data deserves further investigation, the study does not have sufficient statistical power to demonstrate the absence of higher CD28+ positive angiogenic T cells in frailty. In addition, studies to date have only indicated associations between CD3+ angiogenic T cells and vascular health, it is unclear if the putative benefits relate to the CD4+ or CD8+ fraction or both.
To the best of our knowledge, this is the first study designed to examine the separate influences of age and frailty on multiple senescent T cell subsets. The proportion of CD28null T cells was considerably greater in the CD8+ fraction as previously reported (23, 46), few CD4+ T cells were senescent, particularly in the non-frail groups. Small age effects were evident for some outcomes of interest, the proportion of CD4+ and CD8+ T cells negative for CD28 were both higher in the older compared to the young group. There was not clear evidence of progressive changes with increasing age however and the small CD28null age effect was not evident in the CD3+ T cell analysis. Senescent T cells did change considerably in frailty with CD3+CD28null, CD4+CD28null and CD8+CD28null counts and percentages all higher in the frail compared to robust non-frail populations. In the frail group circa half of the CD8+ T cells did not express CD28. Previous studies reporting age effects on CD8+CD28null T cells (46) did not disaggregate healthy and unhealthy ageing, our results indicate that the considerable increase in CD8+CD28null T cells in older individuals is primarily associated with unhealthy ageing. Senescent T cells have utility for monitoring intervention efficacy in older cohorts where there is functional decline and progression into frailty states. This study excluded pre-frail participants. Future work might consider changes in CD28null T cells across the frailty continuum, but in also in cohorts over 85 years, to more fully delineate the transition to a high preponderance of immunosenescent T cells.
The increasing CD28null T cell counts with increasing levels of cognitive impairment are intriguing and to our knowledge the first such report in the literature. This analysis was confined to the older and frail groups, but analyses were adjusted for age and sex. Particularly evident is the increase in CD28 null counts in those with moderate cognitive impairment. The low sample size for this analysis (n=7 with moderate cognitive impairment) likely limited the ability to demonstrate statistical significance in the CD28null percentage analysis. The association is also potentially interesting from a mechanistic viewpoint (47). Further work is needed to delineate the separate associations of frailty and cognitive function with senescent T cell subsets.
Although, the primary focus of this paper was on T cell subsets, we also monitored whole blood haematology changes and monocyte fractions. In previous studies, the neutrophil to lymphocyte ratio and the monocyte to lymphocyte ratio have been proposed as simple indicators of pathological processes, immune system homeostasis and inflammation (48-51). There appears to be a progressive effect of age on monocytes including all monocyte fractions, granulocytes, the granulocyte to lymphocyte ratio (surrogate for neutrophil to lymphocyte ratio) and the monocyte to lymphocyte ratio, with significant differences between the young and non-frail 76+ groups. These monocyte, granulocyte and haematology ratio changes are consistent with innate immune system activation which is known to be intimately linked to inflammaging (52). Sample size may have limited the power to detect monocyte and monocyte fraction increases with frailty. Such changes deserve further attention given the progressive increase with age.
This study has certain limitations. Although both cohorts were 76 years and older, mean age in the frail 76+ group was ~ 3 years greater than the non-frail 76+ group, despite efforts to age-match. This could not explain the increase in angiogenic T cells with frailty as the effect of age across the other 3 groups was a decreasing one. It is unlikely to explain the considerable increase in CD28null T cells in frailty as there was no age effect evident across the older and non-frail 76+ groups. The recruitment of robust 76+ female participants proved difficult hence the sex imbalance in the non-frail 76+ group. This was not completely unexpected and can be partially explained by the higher prevalence of frailty in older women (53). It is an example of the «male-female health-survival paradox,» or “sex-frailty paradox” where women live longer but with more disability than men (54). We adjusted all analyses for sex, though only very limited significant sex differences were observed in the dataset. The limitations due to the sample size of each cohort have already been acknowledged particularly when trying to conclude that no difference exists. It has also been acknowledged that the cohorts measured in this study do not represent the full spectrum of age or frailty status.
In conclusion, this study has demonstrated the potential for both angiogenic and senescent T cells to serve as vascular-relevant biomarkers in ageing and frailty research. There is a need to refine and define a “desirable” angiogenic T cell profile to enhance biomarker potential in diseased and non-diseased aged cohorts. It is not possible at this stage to identify a single vascular-relevant T cell biomarker that can be used in monitoring intervention efficacy across the lifespan in all cohorts. Presently, angiogenic T cells are relevant to interventions involving healthy ageing and senescent T cells relevant in frailty and cognitive decline.
Conflicts: On behalf of all authors, the corresponding author declares that there is no conflicts of interest.
Funding: This work was funded by an Irish Research Council (IRC) Government of Ireland Postgraduate Scholarship to TB, GOIPG/2018/1701. Open Access funding provided by the IReL Consortium.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56.
2. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-7.
3. Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456-66.
4. Wong TY, Massa MS, O’Halloran AM, Kenny RA, Clarke R. Cardiovascular risk factors and frailty in a cross-sectional study of older people: implications for prevention. Age Ageing. 2018;47(5):714-20.
5. Veronese N, Sigeirsdottir K, Eiriksdottir G, Marques EA, Chalhoub D, Phillips CL, et al. Frailty and Risk of Cardiovascular Diseases in Older Persons: The Age, Gene/Environment Susceptibility-Reykjavik Study. Rejuvenation Res. 2017;20(6):517-24.
6. Schaller MS, Ramirez JL, Gasper WJ, Zahner GJ, Hills NK, Grenon SM. Frailty Is Associated with an Increased Risk of Major Adverse Cardiac Events in Patients with Stable Claudication. Ann Vasc Surg. 2018;50:38-45.
7. Aguilar-Navarro SG, Mimenza-Alvarado AJ, Anaya-Escamilla A, Gutierrez-Robledo LM. Frailty and Vascular Cognitive Impairment: Mechanisms Behind the Link. Rev Invest Clin. 2016;68(1):25-32.
8. Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N, et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition study. Stroke. 2013;44(2):388-93.
9. Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338-50.
10. Orkaby AR, Lunetta KL, Sun FJ, Driver JA, Benjamin EJ, Hamburg NM, et al. Cross-Sectional Association of Frailty and Arterial Stiffness in Community-Dwelling Older Adults: The Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 2019;74(3):373-9.
11. Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13:942796.
12. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8.
13. Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother. 2013;9(6):1364-7.
14. Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116(15):1671-82.
15. Weil BR, Kushner EJ, Diehl KJ, Greiner JJ, Stauffer BL, Desouza CA. CD31+ T cells, endothelial function and cardiovascular risk. Heart Lung Circ. 2011;20(10):659-62.
16. Kushner EJ, Weil BR, MacEneaney OJ, Morgan RG, Mestek ML, Van Guilder GP, et al. Human aging and CD31+ T-cell number, migration, apoptotic susceptibility, and telomere length. J Appl Physiol (1985). 2010;109(6):1756-61.
17. Ross M, Ingram L, Taylor G, Malone E, Simpson RJ, West D, et al. Older men display elevated levels of senescence-associated exercise-responsive CD28(null) angiogenic T cells compared with younger men. Physiol Rep. 2018;6(12):e13697.
18. Larbi A, Fulop T. From «truly naive» to «exhausted senescent» T cells: when markers predict functionality. Cytometry A. 2014;85(1):25-35.
19. Teo FH, de Oliveira RT, Mamoni RL, Ferreira MC, Nadruz W, Jr., Coelho OR, et al. Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell Immunol. 2013;281(1):11-9.
20. Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. 2020;134:110887.
21. Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, et al. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology. 1996;88(4):501-7.
22. Ng TP, Camous X, Nyunt MSZ, Vasudev A, Tan CTY, Feng L, et al. Markers of T-cell senescence and physical frailty: insights from Singapore Longitudinal Ageing Studies. NPJ Aging Mech Dis. 2015;1:15005.
23. Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005;40(1-2):81-7.
24. Lopez P, Rodriguez-Carrio J, Martinez-Zapico A, Caminal-Montero L, Suarez A. Senescent profile of angiogenic T cells from systemic lupus erythematosus patients. J Leukoc Biol. 2016;99(3):405-12.
25. LeBrasseur NK, de Cabo R, Fielding R, Ferrucci L, Rodriguez-Manas L, Vina J, et al. Identifying Biomarkers for Biological Age: Geroscience and the ICFSR Task Force. J Frailty Aging. 2021;10(3):196-201.
26. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194-217.
27. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-9.
28. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-95.
29. Hurtig-Wennlof A, Hagstromer M, Olsson LA. The International Physical Activity Questionnaire modified for the elderly: aspects of validity and feasibility. Public Health Nutr. 2010;13(11):1847-54.
30. Weber C, Shantsila E, Hristov M, Caligiuri G, Guzik T, Heine GH, et al. Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the European Society of Cardiology (ESC) Working Groups «Atherosclerosis & Vascular Biology» and «Thrombosis». Thromb Haemost. 2016;116(4):626-37.
31. O’Carroll L, Wardrop B, Murphy RP, Ross MD, Harrison M. Circulating angiogenic cell response to sprint interval and continuous exercise. Eur J Appl Physiol. 2019;119(3):743-52.
32. Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195(6):789-94.
33. Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Mowes B, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35(6):1987-94.
34. Fornasa G, Groyer E, Clement M, Dimitrov J, Compain C, Gaston AT, et al. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunol. 2010;184(10):5485-92.
35. Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology. 1996;88(1):110-5.
36. Kushner EJ, MacEneaney OJ, Morgan RG, Van Engelenburg AM, Van Guilder GP, DeSouza CA. CD31+ T cells represent a functionally distinct vascular T cell phenotype. Blood Cells Mol Dis. 2010;44(2):74-8.
37. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-72.
38. Gupta S, Young T, Yel L, Su H, Gollapudi S. Differential sensitivity of naive and subsets of memory CD4+ and CD8+ T cells to hydrogen peroxide-induced apoptosis. Genes Immun. 2007;8(7):560-9.
39. Alunno A, Ibba-Manneschi L, Bistoni O, Cipriani S, Topini F, Gerli R, et al. Angiogenic T cells in primary Sjogren’s syndrome: a double-edged sword? Clin Exp Rheumatol. 2019;37 Suppl 118(3):36-41.
40. Cavazzana I, Piantoni S, Sciatti E, Fredi M, Taraborelli M, Bonadei I, et al. Relationship between endothelial dysfunction, videocapillaroscopy and circulating CD3+CD31+CXCR4+ lymphocytes in systemic lupus erythematosus without cardiovascular risk factors. Lupus. 2019;28(2):210-6.
41. Manetti M, Pratesi S, Romano E, Bellando-Randone S, Rosa I, Guiducci S, et al. Angiogenic T cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis. PLoS One. 2017;12(8):e0183102.
42. Wilde B, Mertens A, Arends SJ, Rouhl RP, Bijleveld R, Huitema J, et al. Endothelial progenitor cells are differentially impaired in ANCA-associated vasculitis compared to healthy controls. Arthritis Res Ther. 2016;18:147.
43. Zhao P, Miao J, Zhang K, Lv M, Han Q, Zhu P. Circulating Angiogenic T Cells Are Increased in Lupus Nephritis Patients. Med Sci Monit. 2018;24:5384-90.
44. Zhang T. Autoimmunity as a novel mechanism underlying sarcopenia. Aging (Albany NY). 2023;15(9):3221-2.
45. Salaffi F, Di Matteo A, Farah S, Di Carlo M. Inflammaging and Frailty in Immune-Mediated Rheumatic Diseases: How to Address and Score the Issue. Clin Rev Allergy Immunol. 2023;64(2):206-21.
46. Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33(3):267-82.
47. Budamagunta V, Kumar A, Rani A, Bean L, Manohar-Sindhu S, Yang Y, et al. Effect of peripheral cellular senescence on brain aging and cognitive decline. Aging Cell. 2023;22(5):e13817.
48. Mirna M, Schmutzler L, Topf A, Hoppe UC, Lichtenauer M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep. 2021;11(1):18101.
49. Aktas G, Sit M, Dikbas O, Erkol H, Altinordu R, Erkus E, et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Rev Assoc Med Bras (1992). 2017;63(12):1065-8.
50. Bilgin S, Aktas G, Kahveci G, Atak BM, Kurtkulagi O, Duman TT. Does mean platelet volume/lymphocyte count ratio associate with frailty in type 2 diabetes mellitus? Bratisl Lek Listy. 2021;122(2):116-9.
51. Kocak MZ, Aktas G, Duman TT, Atak BM, Kurtkulagi O, Tekce H, et al. Monocyte lymphocyte ratio As a predictor of Diabetic Kidney Injury in type 2 Diabetes mellitus; The MADKID Study. J Diabetes Metab Disord. 2020;19(2):997-1002.
52. Rasa SMM, Annunziata F, Krepelova A, Nunna S, Omrani O, Gebert N, et al. Inflammaging is driven by upregulation of innate immune receptors and systemic interferon signaling and is ameliorated by dietary restriction. Cell Rep. 2022;39(13):111017.
53. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol. 2017;89:30-40.
54. Gordon EH, Hubbard RE. Do sex differences in chronic disease underpin the sex-frailty paradox? Mech Ageing Dev. 2019;179:44-50.
© The Author(s) 2024