G.L. Doza1, S. van Heden2, F. Oliveira Felix1, V. Singh1, C. Beaudart2
1. Department of Biomedical Sciences, Faculty of Medicine, University of Namur, Namur, Belgium; 2. Clinical Pharmacology and Toxicology Research Unit, Namur Research Institute for Life Sciences (NARILIS), Department of Biomedical Sciences, Faculty of Medicine, University of Namur, Namur, Belgium
Corresponding Author: Professor Charlotte Beaudart, Clinical Pharmacology and Toxicology Research Unit (URCP), Namur Research Institute for Life Sciences (NARILIS), Department of Biomedical Sciences, Faculty of Medicine, University of Namur, Namur, Belgium, Email: charlotte.beaudart@unamur.be, ORCID number: 0000-0002-0827-5303
J Frailty Aging 2024;13(3)224-232
Published online May 28, 2024, http://dx.doi.org/10.14283/jfa.2024.47
Abstract
Current interventions targeting sarcopenia are diverse, incorporating a blend of nutritional, exercise, and pharmacological strategies. Although muscle mass, muscle strength, or functional performance typically serve as the primary endpoints, regulatory agencies have recently emphasized integrating Patient-Reported Outcome Measures (PROMs) as primary or secondary outcomes in interventional studies. This shift acknowledges the importance of PROMs and Patient-Reported Experience Measures (PREMs) in assessing intervention effectiveness and aligns with patient-centered healthcare models. The aims of this systematic review are 1) to identify all sarcopenia-designed interventional studies that used PROMs/PREMs as the primary or secondary outcome, 2) to identify the different PROMs/PREMs used within those studies, and 3) to summarize the effects of sarcopenia-designed interventions on PROMs/PREMs of sarcopenic participants. For that, a systematic search of databases (Medline, EMBASE, Review- Cochrane Central of Register of Controlled Trials, and PsychINFO (Via Ovid)) was conducted in September 2023. The review followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement, and the protocol was registered on Open Science Framework (https://osf.io/zxgwm/). The systematic review identified 17 RCTs as sarcopenia-designed interventional studies reporting PROMs. PROMs covered the assessment of various aspects, including quality of life, depressive symptoms, loneliness/social isolation, daytime sleepiness, insomnia impact, and sleep quality/disturbance. Only one sarcopenia-specific PROM, namely the SarQoL, was reported. The effect of sarcopenia-designed interventions on PROMs showed considerable heterogeneity, underscoring the need for standardization in sarcopenia research by developing a Core Outcome Set (COS). COS in sarcopenia studies would ensure consistent and comparable findings, ultimately enhancing the reliability and effectiveness of interventions.
Key words: Patient-reported outcome measure, quality of life, clinical trials, patient-centered care, Sarcopenia.
Introduction
In 2016, sarcopenia was recognised as a disease with an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) code (1). As global life expectancy continues to rise, sarcopenia presents itself as a significant public health challenge (2). The various consequences of sarcopenia, ranging from the development of physical disability to nursing home admission, depression, hospitalisation, and mortality, are anticipated to significantly impact the quality of life for affected individuals (3, 4). Within the context of health systems shifting towards a more patient-centred model of care, it is crucial to consider the impact of diseases, such as sarcopenia, on patients’ quality of life and directly perceived outcomes, (5). In this context, patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs) emerge as invaluable tools for capturing patients’ perspectives on their health and experiences. PROMs and PREMs aim to report on diseases and symptoms, treatment side effects (such as pain, fatigue, or anxiety), functional outcomes (physical, sexual, social, role, emotional, or cognitive functioning), or multidimensional constructs like HRQoL or health utility (6).
Using PROMs and PREMs as endpoints in clinical studies may improve the understanding of patient’s experience by providing information that may not be captured through biomedical methods due to the difficulty of observing certain aspects and their subjective nature (6). This approach may support healthcare professionals and future patients in choosing the most suitable treatment by giving a clearer view of patient’s experiences and identify any unmet needs or areas in healthcare that require improvement (6). Therefore, using PROMs and PREMs in interventional clinical studies on sarcopenia contribute to a more comprehensive understanding of clinically perceived benefits, fostering an assessment of treatment efficacy. Government regulatory agencies, including the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (7, 8), have advocated for the incorporation of PROMs as primary or secondary outcomes in interventional studies. The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group further also recommends using co-primary endpoints, combining a measure of physical performance with PROMs in all phases III clinical trials for sarcopenia (9).
The current approach to managing sarcopenia involves a multifaceted strategy to mitigate its impact on individuals’ health and well-being. Interventions are diverse, incorporating a blend of nutritional, exercise, and pharmacological strategies. Many of these approaches raise numerous questions regarding their effectiveness, particularly in patient-centred management. Although several studies demonstrate improvements in various parameters such as muscle mass, strength, and physical performance, there are still uncertainties about their impact on enhancing patients’ overall HRQoL (10).
This systematic review addresses the need to understand the impact of sarcopenia interventions from patient’s perspective. We therefore aimed 1) to identify all sarcopenia-designed interventional studies that used a PROMs/PREMs as the primary or secondary outcome; 2) to identify the different PROMs/PREMs used within those studies and 3) to summarize the effects of sarcopenia-designed interventions on PROMs/PREMs of sarcopenic participants.
Methods
The 2020 Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (11) has been followed throughout the whole procedure of this systematic review (completed PRISMA checklist available in Appendix 1). The review protocol has been registered on the Open Science Framework (https://osf.io/zxgwm/).
Literature Search
The electronic databases MEDLINE, EMBASE, Review- Cochrane Central of Register of Controlled Trials, and APA PsycINFO (via OVID platform for all the mentioned bibliographic databases) were searched in September 2023 for any study who have used a PROMs/PREMs in a clinical trial aiming at the management of sarcopenia. The search strategy employed for searching in Medline (Ovid) is available in Appendix 2. The search strategy was further adapted to fit the requirements of each database. Additionally, a manual search within the bibliography of relevant papers was performed in order to complete the bibliographic search. Moreover, we also conducted forward references searching of included studies using Web of Science to identify other research that has referenced any article of interest. We also searched on clinical trial registries (www.clinicaltrial.gov) for potential unpublished studies.
Study selection
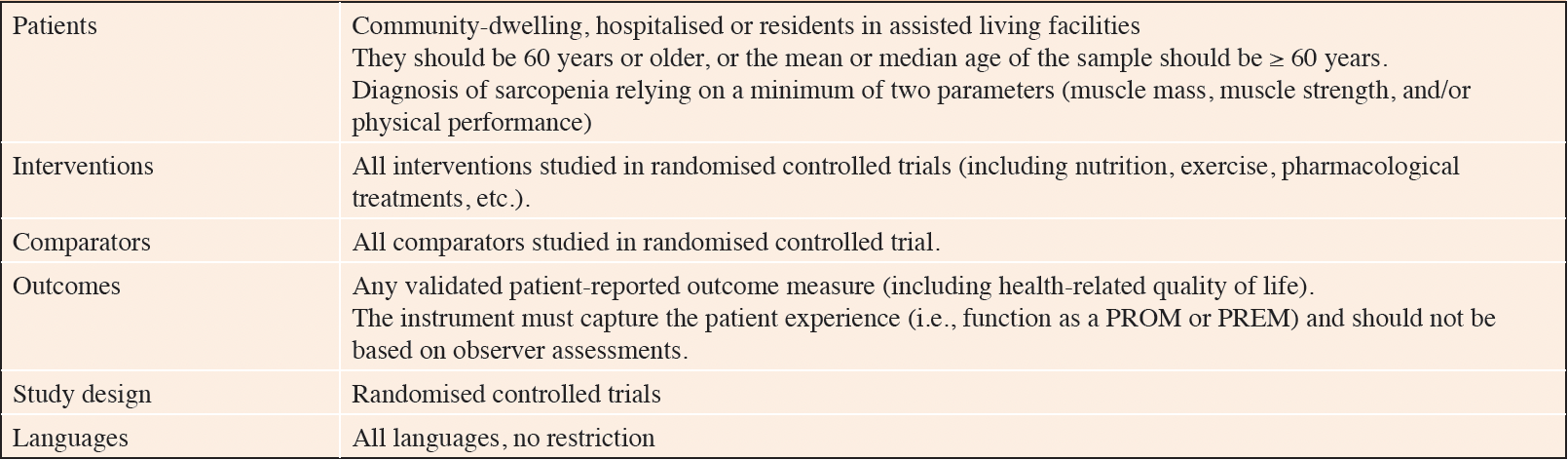
The search results from the electronic sources and hand searching were imported into Covidence software for data management. Covidence is a web-based collaboration software platform that streamlines the production of systematic and other literature reviews (https://support.covidence.org/help/how-can-i-cite-covidence). During the initial screening phase, three reviewers (G.L.D., F.O.F, V.S.) independently assessed the title and abstract of each obtained reference to eliminate articles irrelevant to the systematic review. Rigorous inclusion criteria were applied, as outlined in Table 1. In the subsequent step, the three reviewers individually examined the full text of each article that had not been excluded in the initial stage, selecting studies that fulfilled the inclusion criteria. Any discrepancies in article selection were resolved through discussion and consensus.
Studies were excluded if they included individuals with acute sarcopenia or diagnosed sarcopenia based solely on a single biomarker (e.g., muscle mass only). Additionally, exclusion criteria applied to studies that relied only on a screening tool (e.g., the SARC-F) without further diagnosing the condition, studies that recruited pre-/post-operative hospitalised or disease-specific participants, studies exclusively focused on the diagnosis of sarcopenic obesity, and studies that examined PROMs/PREMs using qualitative research methods.
Data extraction
Data extraction was carried out by three independent reviewers (G.L.D., F.O.F, V.S.) using a standardised form, which had been pretested on a sample of 4 studies.
The following data were extracted: article information (authors, journal name, years of publication, title, country), study description (objective, design, and duration), population characteristics (general description and sarcopenia diagnosis), outcomes (type of PROMs/PREMs), funding details, information on conflicts of interest, and the study’s conclusion. To include as many studies as possible in our systematic review, we systematically contacted authors or co-authors when information was missing in the full-text paper.
Risk of Bias Assessment
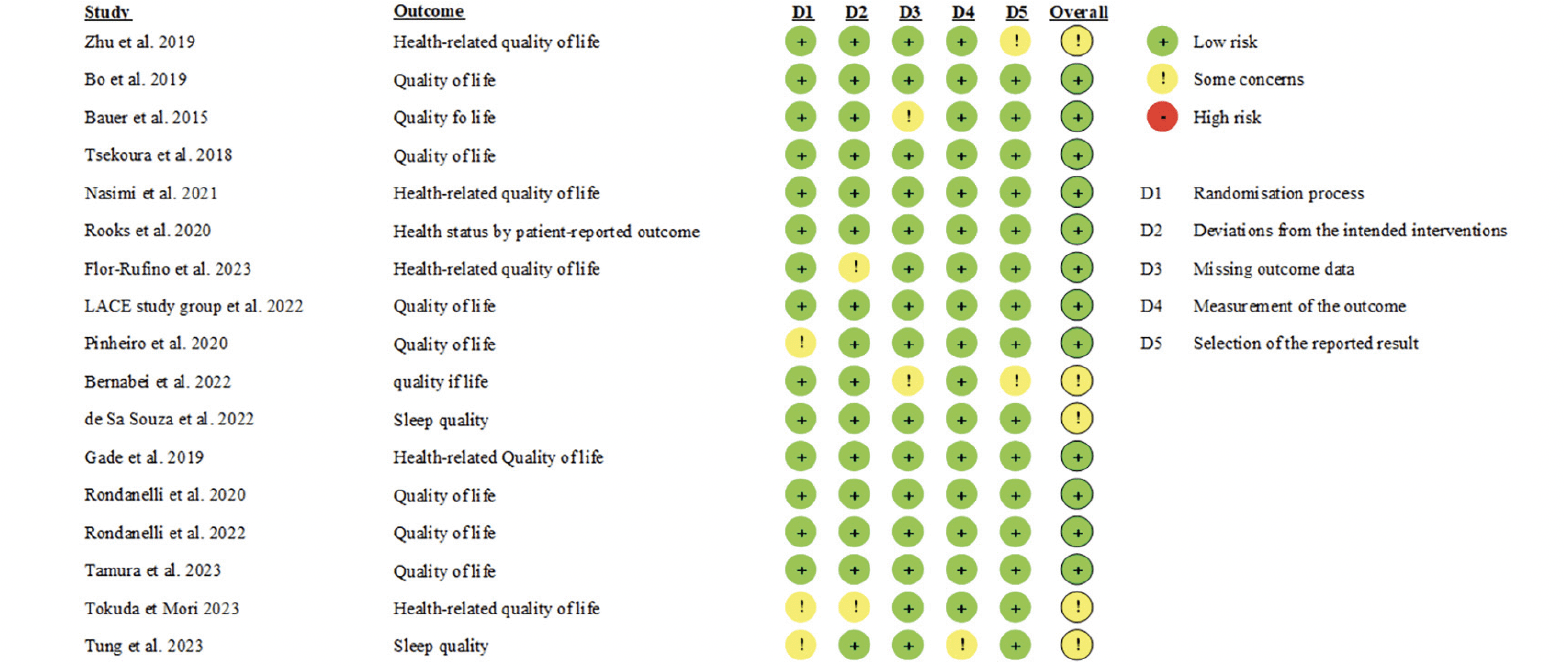
The same three independent reviewers assessed trials’ risk of bias using the Cochrane Risk of Bias Tool 2.0 (12). This tool assesses five domains for each study: randomisation process, deviation from intended interventions, missing outcome data, measurement of the outcome and selection of the reported results. When a study included various PROMs, the focus was primarily on assessing the quality-of-life outcome. For studies featuring a single PROM, the assessment specifically targeted that particular outcome. In case of conflicts, resolution was achieved through consensus, with the option of involving a third party (C.B.).
Data Synthesis
Due to the diverse range of treatments included in this systematic review, conducting a direct comparison using meta-analytic statistics was impossible. Consequently, the findings were summarised and explained narratively.
Results
The search strategy initially identified 2,006 records, with 1,646 records remaining after removing duplicates. Upon screening the titles and abstracts of these records, 77 were considered potentially eligible. Following a thorough evaluation of the full texts of these 77 articles, 60 were excluded and a total of 17 studies met the eligibility criteria and were included in this systematic review. A list of excluded studies and their reasons for exclusion is available on the Open Science Framework deposit (https://osf.io/zxgwm/). Authors from three included paper were contacted by email for additional information or details about their analyses. They all responded positively to our request, providing us enough information to ensure the inclusion of their paper in the current manuscript. Manual search yielded no new reference. Flowchart of study selection is available in Figure 1.
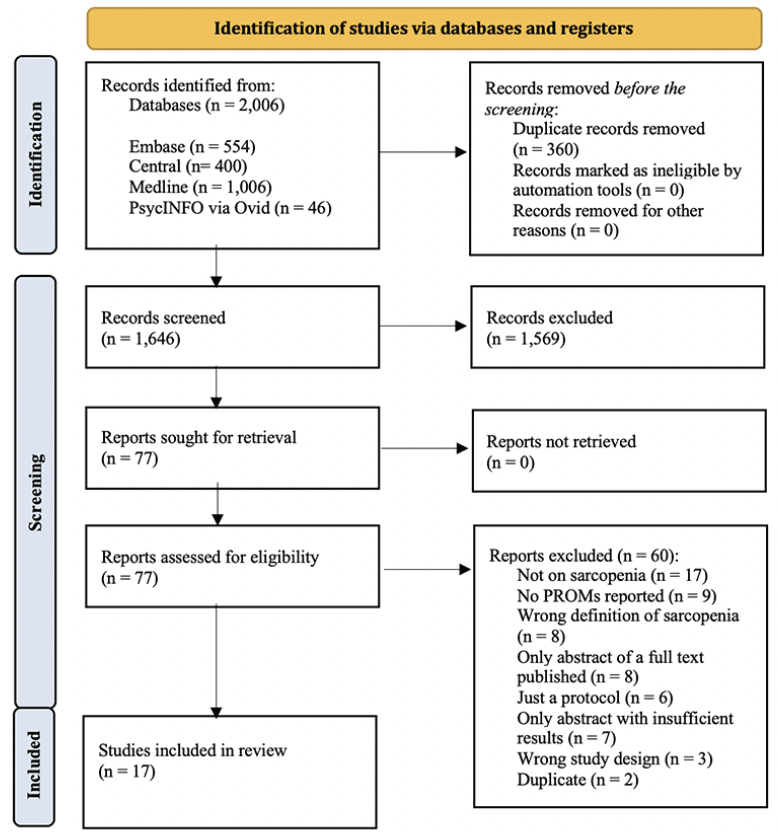
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) flowchart of study selection
Studies characteristics
The 17 randomized-controlled trials (RCTs) included, published between 2015 and 2023, are detailed in Table 3 and Table 4 (13–24). Trial durations ranged widely, spanning 8 (18) to 156 (20) weeks, and study design comprised 2 to 5 groups. The intervention was singular or combined, including medications (n=2, 11.76% (25, 26)), nutritional supplementation (n=6; 35.3% (13–18)), exercise program (n=4, 23.53% (23, 24, 27, 28)) and a combination of exercise and nutritional supplements (n=5, 29.41% (19–22, 29)). At the same time, the control group received isocaloric products/placebos, isocaloric products/placebo combined with an exercise program, or exercise programs alone. The number of participants ranged from 28 (23) to 1519 (20). Only one study exclusively enrolled females (19). Although the targeted population across included studies was sarcopenic, variations were observed in age categories (≥ 60 years, ≥ 65 years, ≥ 70 years) and living conditions encompassing community-dwelling individuals (76.47%), residents of care institutions (11.76%), and candidates for in-patient rehabilitation (5.88%). Regarding sarcopenia diagnosis, 13 studies adhered to a recommended definition by scientific societies, with 4 using AWGS (13, 21, 25, 29), 8 using EWGSOP 1 or 2 (16, 18, 19, 22, 23, 26–28), and 1 using FNIH criteria (20). Other studies used at least two biomarkers to diagnose sarcopenia but did not mention their adherence to a scientific-societies consensual definition.
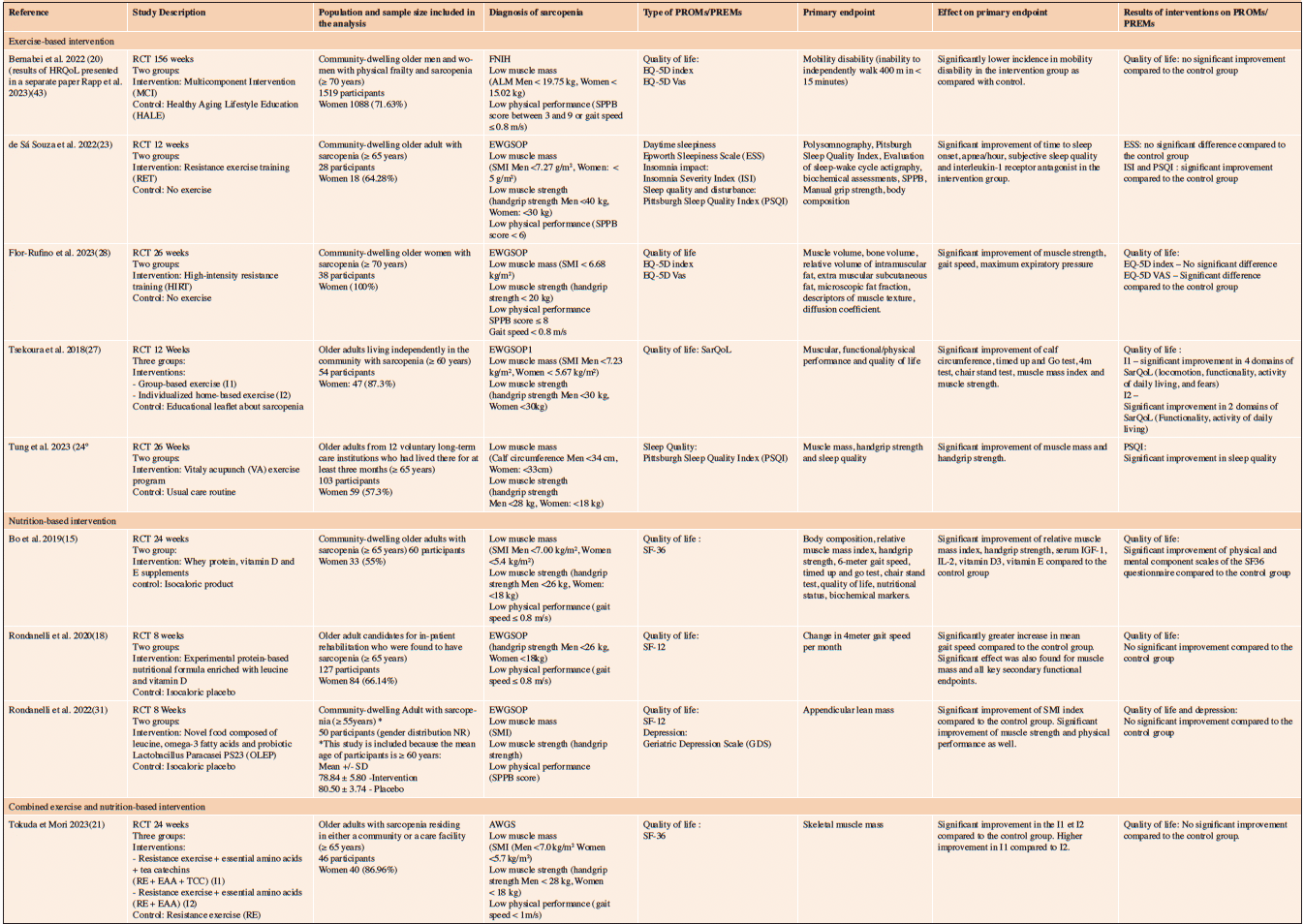
Table 2. General description of the 9 that showed an improvement of the primary outcome following the intervention
RCT: Randomized Controlled Trial, ALM: Appendicular Lean Mass; SPPB: Short Physical Performance Battery; SMI: Skeletal Muscle Index; MCI: Multicomponent Intervention; HALE: Healthy Aging Lifestyle Education ; RET: Resistance exercise training; ESS: Epworth Sleepiness Scale; ISI: Insomnia Severity Index ; PSQI: Pittsburgh Sleep Quality Index; SD: Standard Deviation ;GDS: Geriatric Depression Scale
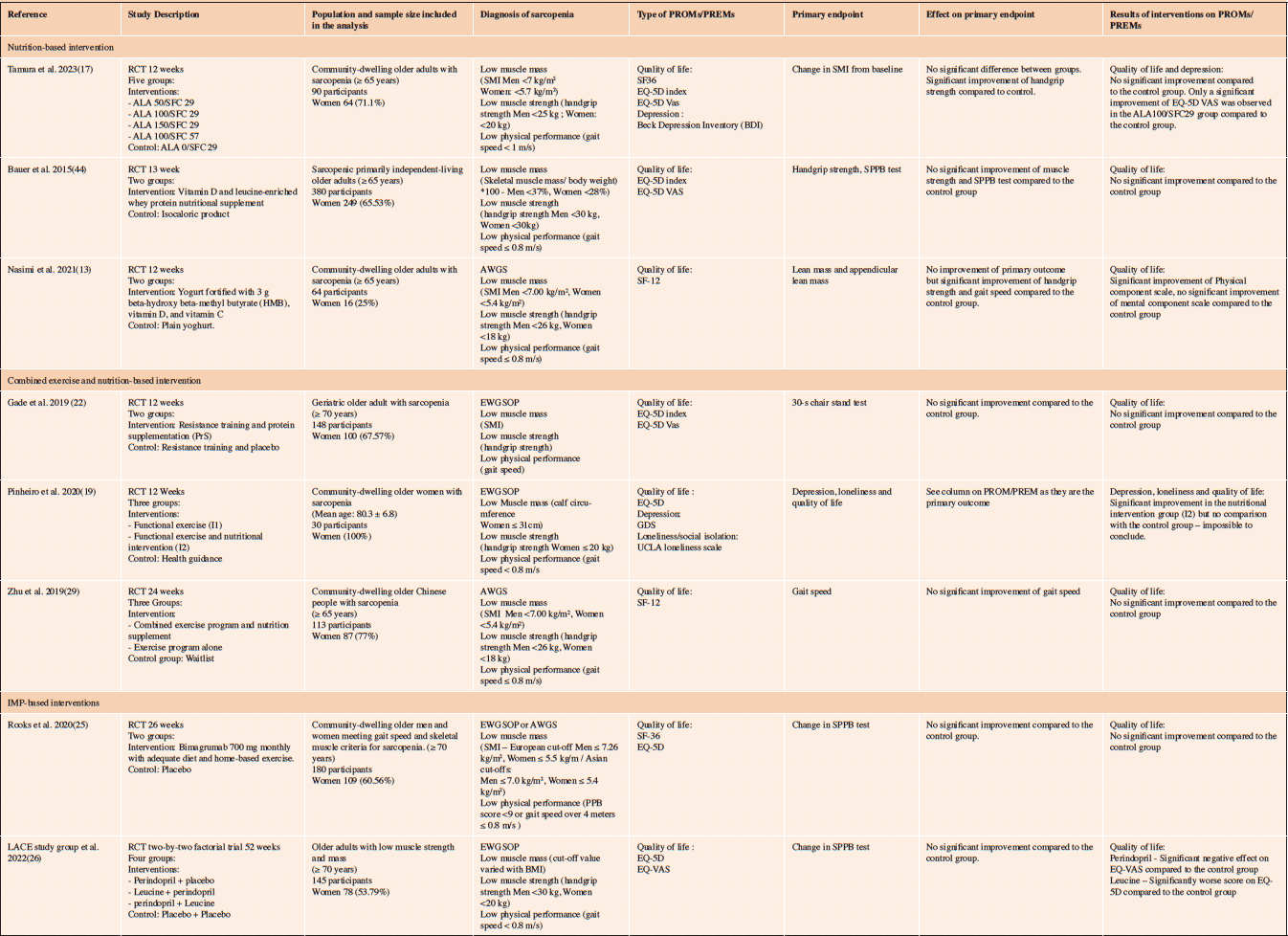
Table 3. General description of the 8 RCTs that showed no improvement of the primary outcome following the intervention
ALA: 5 aminolevulinic acid; SFC: sodium ferrous citrate; BDI; Beck Depression Inventory ;
None of the included studies presented any high risk of bias for any of the investigated domain (Figure 2).
PROMs/PREMs characteristics
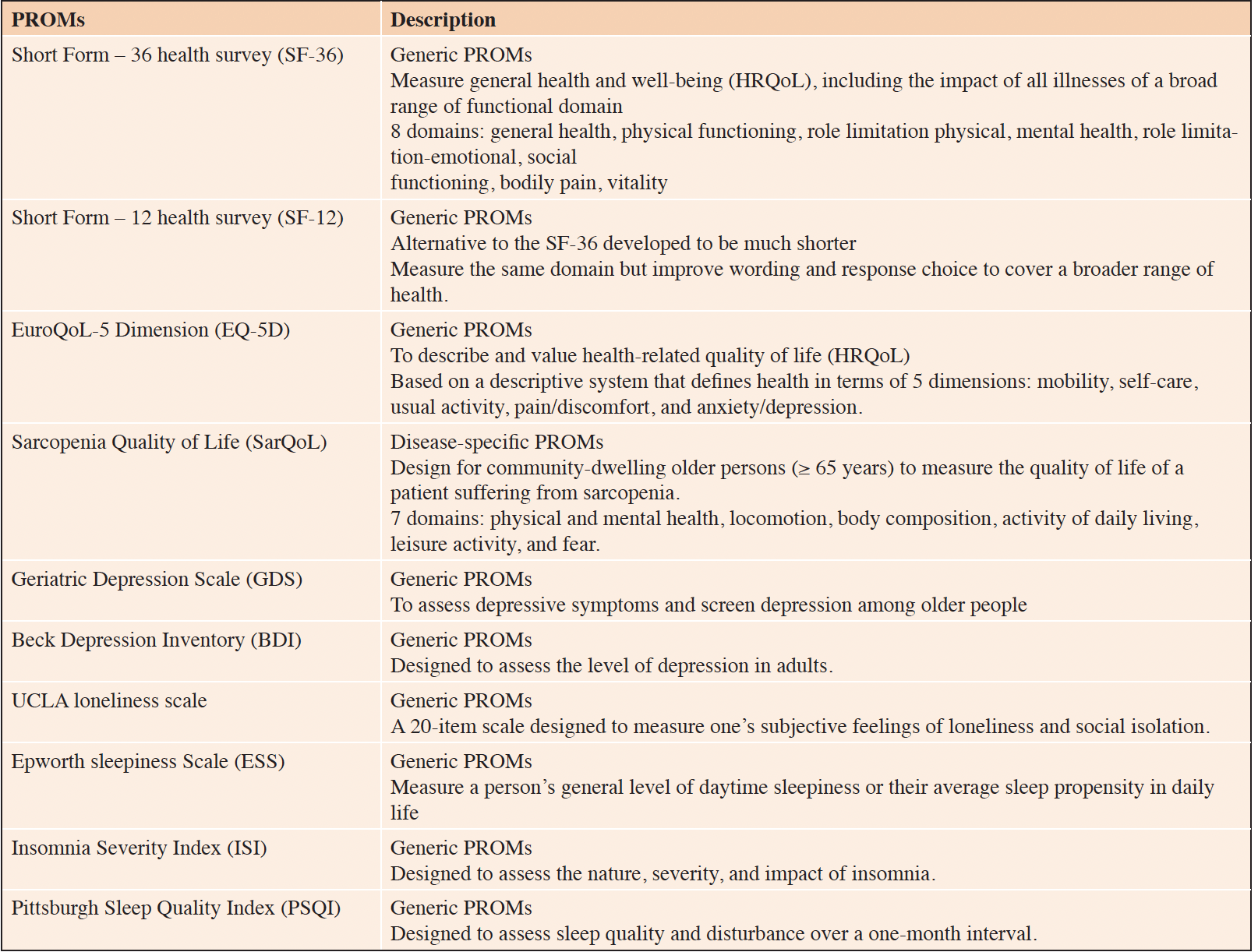
The 17 included studies reported PROMs as outcomes, but none of them reported any PREMs. PROMs utilized in the studies covered assessments of various concepts including HRQoL (SF-12, SF-36, EQ-5D, SarQoL) (13–22, 24–29), depressive symptoms (GDS, BDI) (17, 30), loneliness/social isolation (UCLA) (30), daytime sleepiness (ESS) (23), insomnia impact (ISI) (23), and sleep quality/disturbance (PSQI) (23, 24). The majority of PROMs employed in these studies were generic, with the exception of one study that utilized a sarcopenia-specific PROM called SarQoL (27). Table 2 provides a brief overview of these various PROMs.
PROMs were mainly used as secondary outcomes. Nevertheless, 5 studies listed multiple primary outcomes among which one or more PROMs were listed. Only Pinheiro et al. (19) used exclusively PROMs as primary endpoint (i.e. depression, loneliness and HRQoL).
Results of interventions on primary endpoint
The nature of the primary endpoint varied widely across trials, but in the majority of trials, primary outcome was defined by a physical measurement (i.e. change in gait speed, change in SPBB test, etc.). Further details are provided in Table 3 and Table 4.
Nine RCT reported a significant improvement of the primary endpoint following exercise-based intervention (20, 23, 24, 27, 28), nutrition-based intervention (15, 18, 31) or a combination of exercise and nutrition-based intervention (21) (Table 3). In four of these studies, no further improvement was observed on PROMs (neither HRQoL (18, 20, 21, 31) nor depression (31)). The other five RCTs highlighted both an improvement in the primary endpoint and in PROMs, reflecting that the proposed intervention may be effective in counteracting sarcopenia and its impact on PROMs. De Sa Souza et al. (23) reported a significant effect of a 12-week intervention with resistance exercise training on the Insomnia Severity Index (ISI) and the Sleep Quality Disturbance (PSQI) compared to the control group. Flor-Rufino et al. (28) reported that 26 weeks of high-intensity resistance training may improve muscle strength, gait speed, maximum expiratory pressure alongside the EQ-5D VAS, despite reporting no effect on the EQ-5D utility index. Tsekoura et al. (27) reported that a 12-week group-based exercise and individualized home-based exercise may be effective in improving calf circumference, Time up and Go test, 4-meter gait speed, muscle mass index, muscle strength, and HRQoL PROM, as reported by the SarQoL questionnaire. Tung et al. (24) reported that a 26-week vital acupunch exercise program may be effective in improving muscle mass, muscle strength, and sleep quality as reported by the PSQI PROM. Finally, one nutrition-based intervention, published by Bo et al. (15), using a 24-week intervention with supplements containing whey protein, vitamin D and vitamin E, reported a significant improvement in muscle strength and muscle mass index alongside a significant improvement in HRQoL physical and mental component scales of the SF36 questionnaire.
The other 8 RCTs did not report any significant improvement on the primary endpoint (i.e. SMI, handgrip strength, SPPB test 30-second chair stand test, gait speed) nor on the PROMs either (Table 4). None of the pharmacological intervention-based RCTs (i.e. using Bimagrumab 700mg monthly (25) or perindopril/leucine (26)) reported improvement in the primary endpoint or in PROMs.
Discussion
The objective of this study was to identify sarcopenia-focused interventional studies utilizing PROMs and PREMs as primary or secondary outcomes. This systematic literature review aimed to provide a comprehensive overview of the impact of sarcopenia-designed interventions on outcomes directly considered as relevant by patients suffering from sarcopenia. Seventeen sarcopenia-designed RCTs encompassing interventions such as medication (11.76%), nutritional supplementation (35.3%), exercise programs (23.53%), and a combination of exercise and nutritional supplements (29.41%) were identified. Surprisingly, none of the 17 studies incorporated PREMs but all of them utilized PROMs, mainly as secondary outcomes. The selected PROMs covered diverse concepts, including HRQoL (88.23%), depressive symptoms (17.65%), loneliness/social isolation (5.88%), and sleep quality/disturbance (11.76%).
Out of the 17 sarcopenia-targeting RCTs utilizing PROMs as primary or secondary endpoints, half of them (i.e., 9/17) reported a positive impact of the intervention on sarcopenia parameters (i.e., improvement of muscle mass, muscle strength, or physical performance), and approximately 30% of them (i.e., 5/17) further reported an improvement in PROMs. One hypothesis that could explain these results might be that, with the exception of one study employing a sarcopenia-specific PROM called SarQoL, most reported PROMs were generic. Generic instruments provide a broad assessment of HRQoL in populations and enable comparisons with other conditions, but their generic nature makes them less likely to reflect the impact of the intervention compared to a specific instrument. Specific questionnaires are more sensitive to change. Currently, only three specific PROMs for sarcopenia are documented in the scientific literature, which explains their limited utilization in clinical trials. First, the Age-Related Muscle Loss Questionnaire (ARMLQ – SARCOPRO) has been developed by Evans et al. (32) in 2011 to assess functional impacts of reduced muscle strength. Only the content validity of this PROM has been reported, leaving the other psychometric properties, such as validity, reliability and responsiveness to change unexplored. Second, the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical function item bank, funded by the NIH (33), has been validated for measuring physical function. However, the appropriate context of use and the fit-for-purpose measurement in sarcopenia have not been reported thus far. Thirdly, the SarQoL, a specific quality of life questionnaire for sarcopenia, stands as the only validated PROM currently available. Translated into more than 35 languages and validated across 19 different populations, this PROM is recommended for use in both clinical and research practices (34–36).
The international COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) initiative (37) provides guidelines for selecting the most appropriate PROM in research and clinical settings. COSMIN taxonomy covers key psychometric properties, including reliability (such as reliability, measurement error, and internal consistency), validity (such as content validity, criterion validity, and construct validity), and responsiveness. However, the mere existence of a PROM doesn’t ensure its suitability for measuring a specific concept related to PROMs. In clinical trials targeting sarcopenia management using a PROM as a primary or secondary endpoint, it is crucial to verify that the PROM has been properly developed and validated according to established guidance. In the context of interventional studies, the PROM’s responsiveness to change of a PROM is paramount. The instrument should be sensitive enough to detect any change in the measured concept. For this reason, an ESCEO working group, composed by key experts in the field of clinical trials for sarcopenia, recommends the use of specific PROMs in clinical trials aiming at the management of sarcopenia (9).
The existing diversity in outcome measures underlined in this systematic review also underscores the critical need for standardization in sarcopenia research. The development and adoption of a Core Outcome Set (COS) play a pivotal role in achieving this standardization. The absence of a COS for sarcopenia significantly contributes to the observed variability in PROMs across studies. The COMET (Core Outcome Measures in Effectiveness Trials, https://www.comet-initiative.org/) initiative actively promotes the creation and use of COS, recognizing its numerous advantages (38). COS serves as a valuable tool in averting ineffective interventions and addressing outcome-reporting bias by providing a predefined list of essential outcomes for measurement in Randomized Controlled Trials (RCTs) (39). Its implementation enhances the ability to conduct more consistent systematic reviews or meta-analyses, facilitating robust comparisons across studies and improving the reliability and generalizability of research findings (39). Developing a COS specific to sarcopenia is indispensable not only for establishing uniformity in outcome reporting but also for enhancing the validity and reliability of study results.
This review also brings to light a significant aspect of sarcopenia research: Patient-Reported Experience Measures (PREMs) were not reported in any of the included studies. The underreporting of PREMs in sarcopenia can be attributed to several factors, including researchers’ skepticism, resource limitations, insufficient funding, reluctance to overburden patients, uncertainties about how to utilize PREMs results, and an evident lack of standardization (40). However, addressing the underreporting of PREMs in sarcopenia research requires a broader recognition of the importance of patient perspectives in evaluating intervention effectiveness. Adopting a patient-centered approach is crucial, as neglecting the patient’s experience during an interventional trial can impede shared decision-making in sarcopenia research. Shared decision-making (SDM) explicitly involves patients and clinicians in decisions regarding diagnostic and treatment options (41) by integrating patients’ values and preferences (42). Developing a standardized tool to address this issue could overcome barriers to PREMs implementation and contribute to a more comprehensive and patient-centered approach to sarcopenia studies.
Limitations
This study is constrained by its reliance solely on published literature, potentially overlooking additional interventional studies that are either ongoing or completed but unpublished. Furthermore, our inclusion criteria focused on interventional studies explicitly reporting PROMs or PREMs as primary or secondary outcomes, preventing us from offering a comprehensive prevalence of studies employing such outcomes. Consequently, it remains uncertain whether the use of PROMs is a common or less widespread practice in sarcopenia research. Additionally, the substantial variability in the PROMs used across these studies posed a challenge to conducting a meta-analysis. The differences in outcome measurement instruments between studies created obstacles in synthesizing quantitative data. This emphasizes the critical need for heightened standardization of measurement tools in sarcopenia research. Enhancing standardization would not only facilitate future meta-analyses but also promote comparability across studies, addressing a significant challenge in the current landscape.
Conclusion
In addressing the impact of sarcopenia intervention on PROMs/PREMs, this study identified 17 sarcopenia-designed interventional trials employing diverse strategies such as medication, nutritional supplementation, and exercise. None of these studies used a PREM as primary or secondary outcome. PROMs were exclusively use in those studies covering aspects like HRQoL, depressive symptoms, loneliness and sleep quality. The varied effect on PROMs highlights the need for standardization in sarcopenia research. Developing a Sarcopenia Core Outcome Set is important to ensure consistent, comparable findings, enhancing the intervention’s reliability and effectiveness.
Data availability and transparency: All materials related to this work are freely available on the Open Science Framework deposit.
Conflict of Interest: Charlotte Beaudart is stakeholder of SARQOL SRL, a spin-off of of the University of Liège, in Belgium, in charge of the interests of SarQoL, a specific health-related quality of life questionnaire for sarcopenia. However, she has never received any financial compensation for this role.
Fundings: The authors received no funding for this study.
Authors contribution: C.B. designed the study, the protocol, run the different search strategies. G.L.D, F.O.F and V.S. screened the studies and extracted the data. S.vH. helped in the interpretation of data, manual search and risk of bias assessment. G.L.D and C.B. wrote the manuscript. All authors read and approved the final version of the manuscript.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514
2. Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. 2014. Archives of Public Health. . Archives of Public Health 2014;72:45
3. Veronese N, Demurtas J, Soysal P, et al. Sarcopenia and health-related outcomes: an umbrella review of observational studies. Eur Geriatr Med 2019;10:853–862
4. Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O, Stenroth L. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS One 2017;12:e0169548
5. Weldring T, Smith SMS. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights 2013;6:61
6. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018;Volume 9:353–367
7. Research USD of H and HSFC for DE and, Research USD of H and HSFC for BE and, Health USD of H and HSFC for D and R. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79
8. Oncwp. Reflection Paper on the use of patient reported outcome. 2014
9. Reginster JY, Beaudart C, Al-Daghri N, et al. Update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults. Aging Clin Exp Res . 2021. Springer Science and Business Media Deutschland GmbH, 33: 3–17
10. Park WT, Shon OJ, Kim GB. Multidisciplinary approach to sarcopenia: a narrative review. Journal of Yeungnam Medical Science . 2023. Yeungnam University School of Medicine and College of Medicine, 40: 352–363
11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64
12. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. The BMJ 2011;343
13. Nasimi N, Sohrabi Z, Dabbaghmanesh MH, et al. A Novel Fortified Dairy Product and Sarcopenia Measures in Sarcopenic Older Adults: A Double-Blind Randomized Controlled Trial. J Am Med Dir Assoc 2021;22:809–815
14. Bauer JM, Verlaan S, Bautmans I, et al. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Med Dir Assoc 2015;16:740–747
15. Bo Y, Liu C, Ji Z, et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clinical Nutrition 2019;38:159–164
16. Rondanelli M, Opizzi A, Antoniello N, et al. Effect of essential amino acid supplementation on quality of life, amino acid profile and strength in institutionalized elderly patients. Clin Nutr 2011;30:571–7
17. Tamura Y, Kaga H, Abe Y, et al. Efficacy and Safety of 5-Aminolevulinic Acid Combined with Iron on Skeletal Muscle Mass Index and Physical Performance of Patients with Sarcopenia: A Multicenter, Double-Blinded, Randomized-Controlled Trial (ALADDIN Study). Nutrients 2023;15
18. Rondanelli M, Cereda E, Klersy C, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle 2020;11:1535
19. Pinheiro HA, Cerceau VR, Pereira LC, Funghetto SS, Menezes RL de. Nutritional intervention and functional exercises improve depression, loneliness and quality of life in elderly women with sarcopenia: a randomized clinical trial. Fisioterapia em Movimento 2020;33:e003332
20. Bernabei R, Landi F, Calvani R, et al. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ 2022;377
21. Tokuda Y, Mori H. Essential Amino Acid and Tea Catechin Supplementation after Resistance Exercise Improves Skeletal Muscle Mass in Older Adults with Sarcopenia: An Open-Label, Pilot, Randomized Controlled Trial. Journal of the American Nutrition Association 2023;42:255–262
22. Gade J, Beck AM, Andersen HE, et al. Protein supplementation combined with low-intensity resistance training in geriatric medical patients during and after hospitalisation: a randomised, double-blind, multicentre trial. British Journal of Nutrition 2019;122:1006–1020
23. de Sá Souza H, de Melo CM, Piovezan RD, et al. Resistance Training Improves Sleep and Anti-Inflammatory Parameters in Sarcopenic Older Adults: A Randomized Controlled Trial. Int J Environ Res Public Health 2022;19
24. Tung HT, Chen KM, Chou CP, Belcastro F, Hsu HF, Kuo CF. Acupunch Exercise Improved Muscle Mass, Hand Grip Strength, and Sleep Quality of Institutional Older Adults with Probable Sarcopenia. https://doi.org/101177/07334648221141413 2022;42:888–897
25. Rooks D, Swan T, Goswami B, et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial. JAMA Netw Open 2020;3:e2020836
26. Witham MD, Adamson S, Avenell A, et al. Leucine and perindopril to improve physical performance in people over 70 years with sarcopenia: the LACE factorial RCT. Efficacy and Mechanism Evaluation 2022;9:1–82
27. Tsekoura M, Billis E, Tsepis E, et al. The effects of group and home-based exercise programs in elderly with sarcopenia: A randomized controlled trial. J Clin Med 2018;7:480
28. Flor-Rufino C, Barrachina-Igual J, Pérez-Ros P, Pablos-Monzó A, Martínez-Arnau FM. Resistance training of peripheral muscles benefits respiratory parameters in older women with sarcopenia: Randomized controlled trial. Arch Gerontol Geriatr 2023;104:104799
29. Zhu LY, Chan R, Kwok T, Cheng KCC, Ha A, Woo J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: A randomized controlled trial. Age Ageing 2019;48:220–228
30. Pinheiro MB, Oliveira J, Bauman A, Fairhall N, Kwok W, Sherrington C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. International Journal of Behavioral Nutrition and Physical Activity . 2020. Int J Behav Nutr Phys Act, 17
31. Rondanelli M, Gasparri C, Barrile GC, et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2022;14
32. Evans CJ, Chiou C-F, Fitzgerald KA, et al. Development of a new patient-reported outcome measure in sarcopenia. J Am Med Dir Assoc 2011;12:226–33
33. Zeeshan B, David C, Jensen S, Shaunfield S. Development of a context of use and PROMIS Physical Function outcome assessment for patients with sarcopenia.
34. Beaudart C, Biver E, Reginster J-YJ-Y, et al. Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects: the SarQoL. Age Ageing 2015;44:960–966
35. Beaudart C, Biver E, Reginster J-Y, et al. Validation of SarQoL®, a specific health-related quality of life questionnaire for sarcopenia. J Cachexia Sarcopenia Muscle 2018;8:238–244
36. Beaudart C, Reginster J-Y, Amuthavalli Thiyagarajan J, et al. Measuring health-related quality of life in sarcopenia: summary of the SarQoL psychometric properties. Aging Clin Exp Res 2023
37. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010;63:737–745
38. Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: Version 1.0. Trials . 2017. BioMed Central Ltd., 18: 1–50
39. Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132
40. Shunmuga Sundaram C, Campbell R, Ju A, King MT, Rutherford C. Patient and healthcare provider perceptions on using patient-reported experience measures (PREMs) in routine clinical care: a systematic review of qualitative studies. J Patient Rep Outcomes . 2022. Springer Science and Business Media Deutschland GmbH, 6
41. Damman OC, Jani A, de Jong BA, et al. The use of PROMs and shared decision-making in medical encounters with patients: An opportunity to deliver value-based health care to patients. J Eval Clin Pract 2020;26:524–540
42. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns 2015;98:1172–1179
43. Rapp T, Sicsic J, Ronchetti J, Cicchetti A. Preventing autonomy loss with multicomponent geriatric interventions: A resource-saving strategy? Evidence from the SPRINT-T study. SSM Popul Health 2023;24
44. Bauer JM, Mikušová L, Verlaan S, et al. Safety and tolerability of 6-month supplementation with a vitamin D, calcium and leucine-enriched whey protein medical nutrition drink in sarcopenic older adults. Aging Clin Exp Res 2020;32:1501–1514
© The Author(s) 2024