J.-B. Gauvain1, S. Mandigout2, M. Pambet1, M. Monseu1, P. Gillain4, J. Gautier3, C. Annweiler3, F. Puisieux4
1. Court Séjour Gériatrique, Department of Geriatric Medicine, CHU d’Orléans, 14 Av. de l’Hôpital, F-45067 Orleans, France; 2. Univ. Limoges, HAVAE, UR 20217, F-87000 Limoges, France; 3. Department of Geriatric Medicine, Centre de Recherche sur l’Autonomie et la Longévité (CeRAL), UPRES EA 4638, University of Angers, 4 rue Larrey, F-49933 Angers, France; 4. Gerontology Clinic and Department of Internal Medicine and Geriatrics, Hôpital gériatrique les Bateliers, 23 Rue des Bateliers, University of Lille, F-59037 Lille, France.
Corresponding Author: Prof Mandigout Stéphane, University of Limoges, HAVAE UR20217, 123 Av Albert Thomas, 87060 Limoges, France, Stephane.mandigout@unilim.fr, Tel: +33 6 45 27 39 86
J Frailty Aging 2024;13(3)240-247
Published online June 11, 2024, http://dx.doi.org/10.14283/jfa.2024.53
Abstract
BACKGROUND: The study investigates the correlation between muscle mass and physical activity level measured objectively and subjectively in older adults who fall or are at high risk of falling.
METHODS: FITNESS (Fall Interest to Target Newly Sarcopenic Society) is a multi-center (French university hospitals of Angers, Lille, Limoges and Orléans), cross-sectional, observational study of routine care within a French multidisciplinary hospital consultation. Inclusion criteria were ≥ 75 years old, living at home and consulting for fall or gait disorder. A standardized geriatric assessment, muscle mass evaluation by impedancemetry, physical activity by continuous actimetry (5 days) and Incidental and Planned Exercise Questionnaire (IPEQ) were performed at patient inclusion.
RESULTS: 170 people aged 75 and over were included in the FITNESS study (mean age 82.9 ± 4.7 years, women 72.9%). Muscle mass (whole body and lower limbs) correlated with active energy expenditure (AEE, ρ whole body = 0.32, p-value < 0.001; ρ lower limbs = 0.25, p-value = 0.003), but not with number of daily steps, nor with IPEQ score. Multivariate analysis of whole-body muscle mass showed a positive and significant association with AEE and albumin levels and for lower limb muscle mass, a positive association with AEE and Charlson.
CONCLUSION: This study suggests that in the particular population of older adults who fall and/or are at high risk of falling, loss of muscle mass correlates with reduced physical activity. So subjects who fall or at high risk of falling constitute a special group for whom the fight against sedentary lifestyles and the maintenance of physical activity should be a dual priority.
Key words: Frailty, muscle mass, physical activity, active energy expenditure, multidisciplinary fall consultation.
Introduction
As we age, the likelihood of experiencing a fall increases. In fact, 30% of adults aged over 65 years fall annually, with this figure rising to 50% for those over 80 years old (1). It is also worth noting that half of all fallers will fall again within a year. Falls are one of the main reasons why hospital emergency services are called. Falls can cause loss of functional independence, which in turn can lead to institutionalisation (2). This public health issue demands a systematic approach to identifying at-risk subjects during any consultation with older people (OP) (2), since fallers or their family trivialize past falls. The latest international recommendations pragmatically propose a stratification according to the risk level of falling (low, intermediate, high). This proposed assessment offers the advantage of suggesting an adapted etiological and strategic approach for the three groups of subjects identified (2). The multifactorial nature of falls has led to the implementation of multidimensional assessments for patients at high risk of falling, either in consultation or at day hospitals. The first such assessments were conducted approximately thirty years ago in France (3). In a community setting, a multifactorial approach tailored to each individual has been demonstrated to enable secondary prevention, resulting in a significant 24% reduction in the risk of falls (4). Furthermore, there is a strong correlation between the risk of falling and reduced walking speed, as well as frailty syndrome. This syndrome is also linked to repeated falls and severe falls. Conversely, a fall can serve as a marker of frailty (5). So the reversible nature of frailty justifies an active, personalized approach aimed at reversing it (6).
Conversely, sarcopenia is an essential component of the concept of frailty (6). Consistent data suggest that sarcopenia is a risk factor for falls (7). Sarcopenia is a condition characterized by age-related loss of skeletal muscle mass, strength and function (8). In 2019, a consensus of European experts proposed a definition for sarcopenia in the older adults (9). A reduction in muscle strength is a prerequisite for the diagnosis of probable sarcopenia. A reduction in mass serves to confirm this diagnosis, while a disturbance in a physical performance test allows for the assessment of the severity of the condition (walking speed ≤ 0.8 m.sec-1, Time Up and Go ≥ 20 sec, SPPB ≤ 8 points). In non-dependent OP, a multi-domain assessment can be employed to stratify the level of vulnerability and anticipate events such as falls or institutionalization (10).
In OP, physical performance is closely associated with muscle mass, and even more so with muscle strength (11). However, data from the general population, particularly in France, suggest that the majority of OP have a level of physical activity below that recommended and a high level of sedentary time (12). Nevertheless, without reaching recommended levels, a slight increase in physical activity could have beneficial effects on the ability to carry out the gestures of daily life, thus preserving functional independence (10). This is of major importance for older patients suffering from falls, most of whom are frail and have a low level of physical activity (6).
It is also well established that adapted physical exercise can reduce the risk of falls (13). However, in the real world, long-term adherence to these recommendations is low and, for many OP, illusory (14). What’s more, especially in frail older patients, it’s important to bear in mind that physical activity includes not just adapted exercise, but any bodily movement produced by skeletal muscles and resulting in energy expenditure (occupational, sports, conditioning, household, or other activities) (2). In a recent article, Ramsey et al. (15) remind us that every step counts and that the number of daily steps is the objective parameter most consistently associated with clinical outcomes. However, physical activity recommendations for the OP are not defined based on step count but rather on energy expenditure (16).
Consequently, falls, low levels of physical activity, sarcopenia and frailty syndromes interact in a mutually reinforcing manner. Paradoxically, in the older population with falls, there is a paucity of data to confirm that sarcopenia and physical activity level remain significantly associated (15). The rationale for conducting this study in an older population who are at high risk of falling is to investigate the relationship between objectively and subjectively measured physical activity and muscle mass.
Methods
Study population
The «Fall Interest to Target Newly Older Sarcopenic Society» (FITNESS) cohort is the result of collective work carried out in 4 French university hospitals (Angers, Lille, Limoges and Orléans). These hospitals are equipped with a multidisciplinary falls consultation service. Participants were referred by their general practitioner (GP), a specialist, or an emergency physician, and all underwent a Comprehensive Geriatric Assessment (CGA). The study was observational, descriptive, cross-sectional and multicenter. Subjects were included between October 2016 and July 2021 in routine ambulatory care during a multidisciplinary consultation for a fall or gait disorder.
To be eligible for inclusion, patients had to be at least 75 years old, living at home and consulting for a fall or gait disorder. Criteria for non-inclusion were opposition to study participation, a Mini-Mental State Examination (MMSE) score (17) < 14/30, significant loss of inde-pendence with an Activities of Daily Living (ADL, Katz Index) score (18) < 4/6 or a medically unstable state.
Patients eligible for FITNESS were informed, in comprehensible terms, of the study’s objectives, their right to refuse to participate and the possibility of withdrawing at any time. If they had not expressed any opposition, a letter of information was given to the participant and their carer before they signed a non-opposition form, which was recorded in the patient’s medi-cal file. The protocol was approved by the independent ethics committee at Orleans University Hospital (Orléans, France; reference: 2016-02, dated April 26, 2001) and the French National Consultative Committee on Information Processing in Medical Research at the French Ministry of Research (CCTIRS, Paris, France; reference: 16.522, dated July 12, 2016).
Data collection and processing
• Physical activity assessment: Physical activity was measured objectively by wearing an activity tracker (Armband, SenseWear®) for 5 consecutive days. The armband was fitted by the nurse, who, in the presence of the primary caregiver, explained the monitoring instructions and the need to return it 5 days later. It was worn on the left arm behind the triceps. The nurse checked that the cuff did not slip down the arm and that the strap was not too tight. The patient was instructed to wear the cuff day and night, removing it only for hygiene purposes (a maximum of 1 hour per day was recommended). This recording was used to measure the number of daily steps over 5 days and active energy expenditure (kcal.day-1), (AEE) which is the modifiable component of total energy expenditure (TEE) derived from all activities, both volitional and non-volitional (19). Sedentary time includes all physical activities with low energy expenditure (< 1.6 MET). In practice, in our study, the Armband measurement recorded all activity above 1.6 METs, including moderate activity time. Physical activity assessment was also measured using the IPEQ questionnaire (activity score in hours/week) (20).
• Geriatric assessment: It was carried out by a geriatric physician, assisted by the consultation nurse and the main caregiver, who was present most of the time. Data was collected in two stages. The first phase took place in the geriatric consultation. The assessment focused on socio-demographic and clinical variables related to the risk of falling. The second consultation, organized remotely by appointment, enabled the assessment of the risk of falling to be completed on the basis of the risk points identified during the first visit. At the end of the consultation, patients received a summary of the recommendations, prioritized and adapted to their state of health.
• Measurement of muscle mass and strength: Suspicion of sarcopenia was identified by measuring muscle strength (handgrip < 27 Kg in men, < 16 Kg in women) and the chair lift test 5 times (> 15 sec). Whole-body muscle mass and lower-limb muscle mass were measured by impedancemetry (MC-780MA S, Tanita, The Netherlands) and expressed as a percentage of total body weight.
• Other variables collected: Assessment of functional independence for basal activities of daily living was performed via the Activity of Daily Living (ADL) scale (18). Health status was assessed by looking for cognitive disorders (MMSE score < 27/30) (17) measurement of a comorbidity index Charlson score (21), serum albumin and vitamin D deficiency (25(OHD) < 30 ng.ml-1), as well as the presence or absence of polymedication (≥ 5 drugs/day). A correspondence list between patient order number and patient name was kept in the office of the research manager at each center. For each participant, the variables collected were transcribed in coded form in a personal, anonymized online observation notebook.
• Statistical analysis: Participants’ clinical characteristics were described as numbers and percentages in the case of qualitative variables, and as means ± standard deviation, or as medians (Quartiles 25-75) according to distribution in the case of quantitative variables. To meet the main objective of the study, Spearman correlation coefficients were calculated to measure correlations between muscle mass (whole body and lower limbs) and physical activity level (energy expenditure, number of steps and IPEQ score). These non-parametric tests were chosen because of the non-Gaussian distribution of the variables.
Univariate and then multivariate linear regression models were run to determine the association between whole-body muscle mass and clinical characteristics, including physical activity level, and subsequently between lower limb muscle mass and these same clinical characteristics.
P-values < 0.05 were considered significant. All analyses were performed using SAS software® Version 9.4 (SAS Institute Inc.). The initial calculation of the number of subjects required was 200 patients.
Results
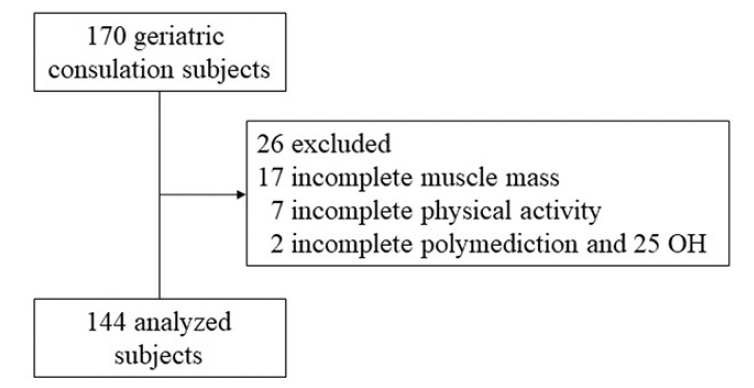
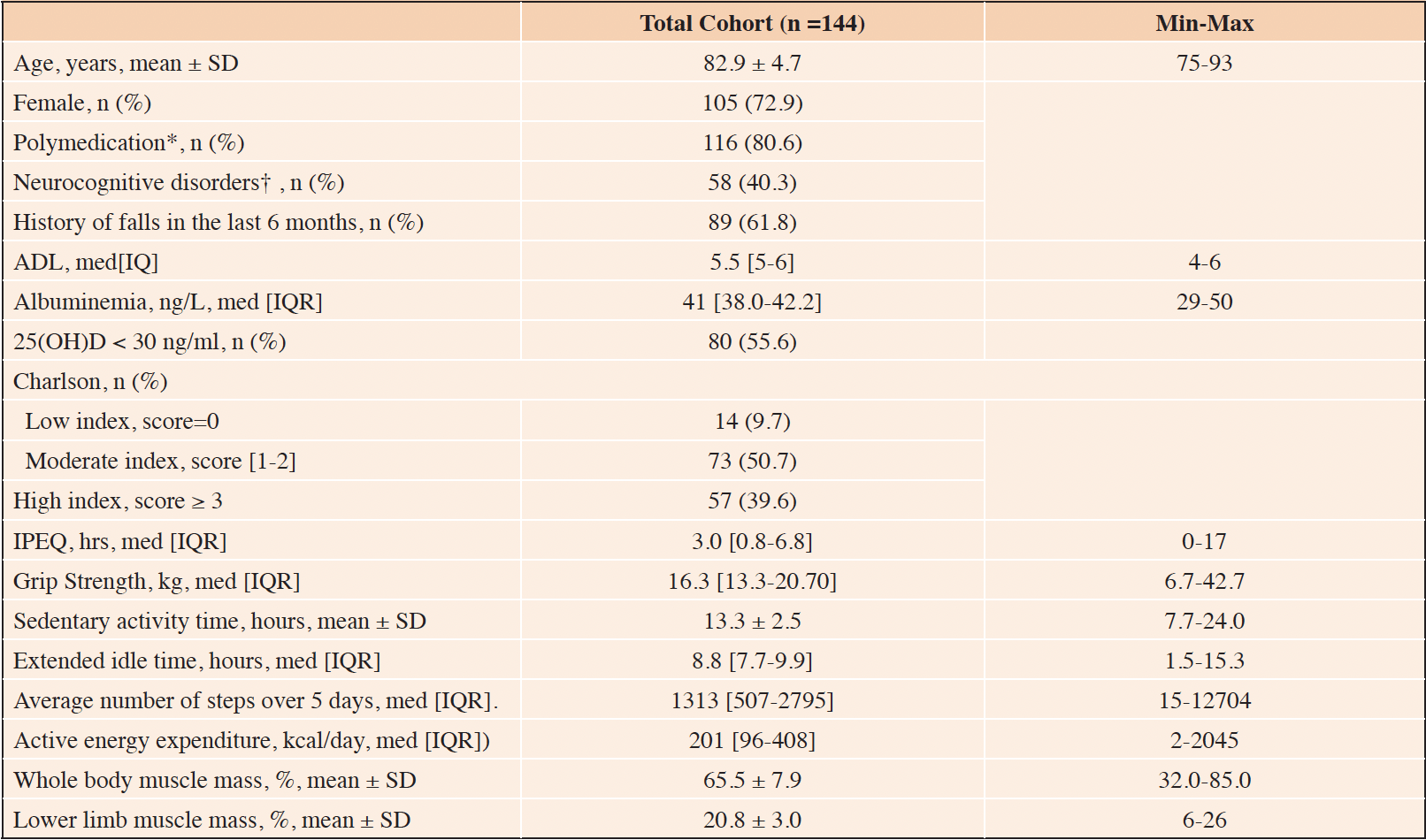
Between October 2016 and July 2021, 170 patients were included in the study, of whom 144 could be analyzed (Figure 1). Indeed, 26 patients had to be excluded due to incomplete data. Their clinical characteristics are reported in Table 1. The average age of the participants was 82.9 ± 4.7 years and 105 were women (72.9%).
ADL: Activities of Daily Living; IPEQ: Incidental and Planned Exercise Questionnaire; IQR: Interquartile Range; med: median; MMSE: Mini Mental State Examination; SD: Standard Deviation; * ≥ 5 medications/day;† MMSE Score <27/30 ;
In our study, 38.2% of the population had not fallen but were at high risk of falling. Eighty-nine patients (61.8%) had experienced at least one fall in the previous six months, and 49 (34.0%) had experienced at least two. Loss of at least one ADL point was found at the time of inclusion in 27.8% of cases (n= 40). The majority of patients, 84.6% of men (n= 33) and 79.0% of women (n= 83), had grip-test muscle strength below the threshold value for suspected sarcopenia (handgrip < 27 Kg in men, < 16 Kg in women). The time taken to perform the 5 chair lifts was abnormal (> 15 sec) in 66.7% of men (n= 26) and 67.6% of women (n= 71). Mean whole-body muscle mass was 65.5 ± 7.9% and 20.1 ± 3.0% for the lower limbs.
The median number of daily steps over 5 days was 1313 (507 – 2795). Active energy expenditure was less than 201 kcal.day-1 for half the population (201 (96 – 408)), and mean sedentary time was 13.3 ± 2.5 hours. Fifty percent of the population had a sedentary time of less than 8.8 hours (7.7 – 9.9). The median number of hours of physical activity/week (IPEQ score) was 3 hours (0.8 – 6.8).
Muscle mass (whole body and lower limbs) correlated with physical activity level measured by active energy expenditure (AEE) (ρ= 0.32, p-value < 0.001 and ρ= 0.25, p= 0.003, respectively), (table 2). No significant correlation was found between muscle mass (whole body and lower limbs) and number of steps, or between muscle mass (whole body and lower limbs) and IPEQ score (Table 2).
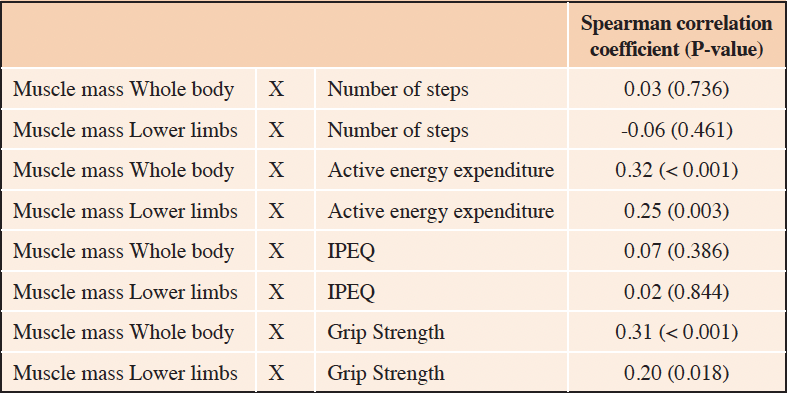
Table 2. Correlations between muscle mass and level of physical activity assessed by different measures (number of steps, active energy expenditure and IPEQ) (n=144)
Multivariate analyses, presented in Table 3, reveal an association between whole-body muscle mass and AEE (β= 0.56; CI 95%= (0.20; 0.92)). The association between lower limb muscle mass (Table 4) and AEE also emerged significantly (β=0.16; CI 95% = (0.01; 0.31)). Female gender, albumin level, serum vitamin D level and level of comorbidities were also associated with lower limb muscle mass.
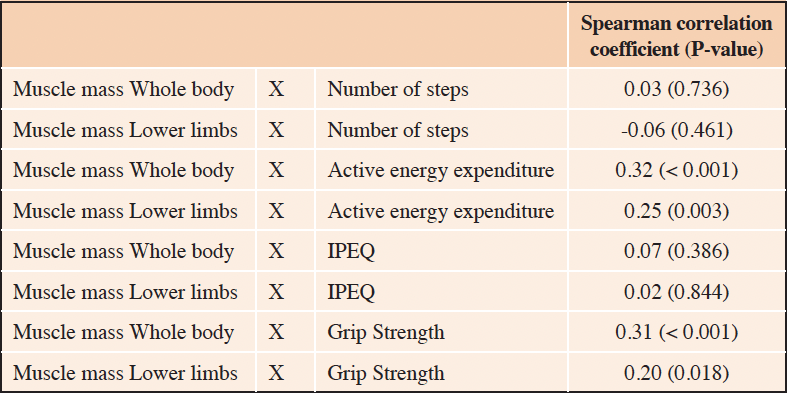
Table 3. Linear regressions concerning the association between whole body mass (dependent variable) and clinical characteristics including physical activity level (independent variables) (n=144)
ADL: Activities of Daily Living; * ≥ 5 medications/day;† MMSE (Mini Mental State Examination) score <27/30
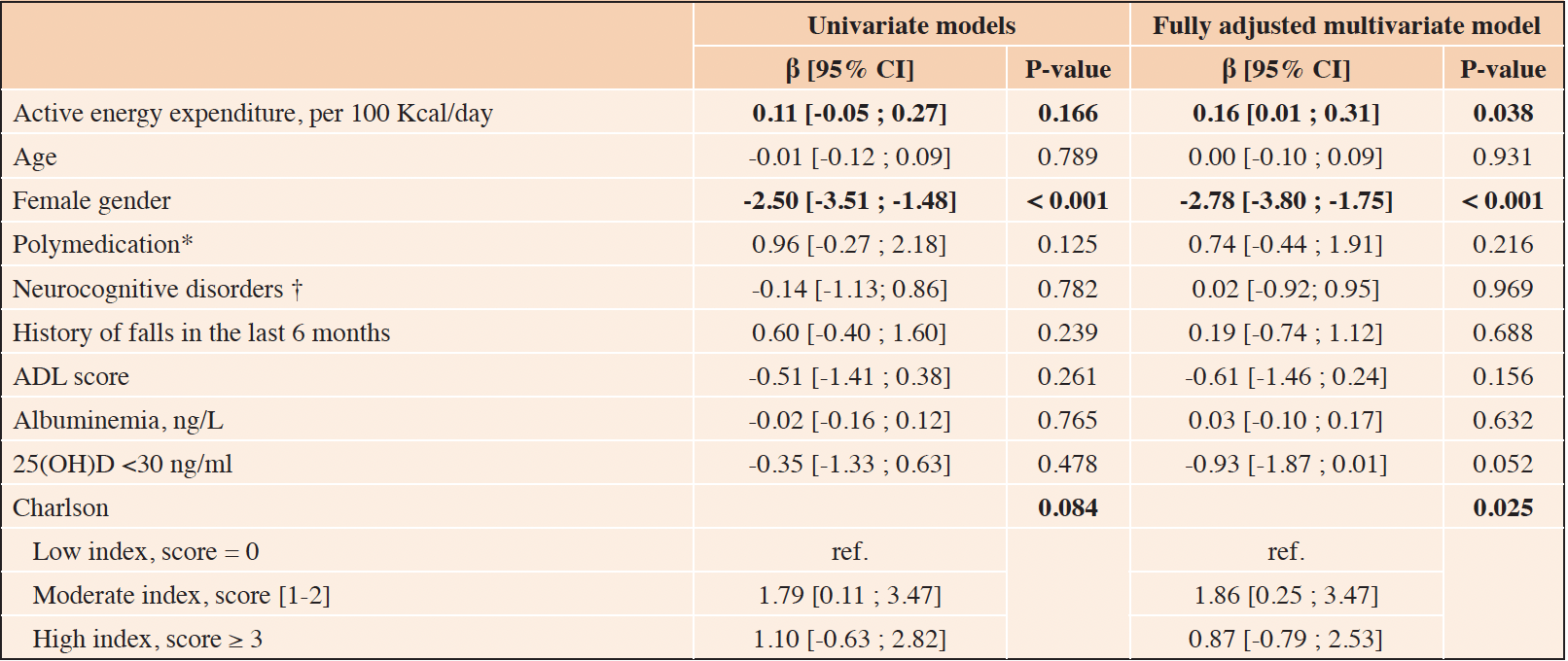
Table 4. Linear regressions concerning the association between lower limb mass (dependent variable) and clinical characteristics including physical activity level (independent variables) (n=144)
ADL: Activities of Daily Living; * ≥ 5 medications/day;† MMSE (Mini Mental State Examination) score <27/30
Discussion
In a population of 144 OP consulting for falls or gait disorders, whole-body and lower-limb muscle mass correlated with AEE, but not with the number of daily steps measured by actimetry, nor with the IPEQ score. Multivariate analysis of whole-body muscle mass showed a positive and significant association with AEE and albumin levels, and a negative association with female gender and vitamin D deficiency. Multivariate analysis of lower limb muscle mass showed a positive association with AEE and Charlson, and a negative association with female gender and vitamin D deficiency. To our knowledge, these data, which concern an older population at high risk of falling, are original. They suggest that even in this frail population with low physical activity and at risk of loss of mobility and functional dependence, maintaining daily physical activity is associated with better preservation of muscle mass.
In our study, 38.2% of subjects had not experienced a fall in the previous six months. These individuals were referred to the multidisciplinary fall consultation due to gait and balance disorders, which are significant risk factors for falls and often serve as predictors of future falls (2). Our study population is comparable to other geriatric populations exposed to falls (22) particularly for the prevalence of neurological disorders (40.3% in our population) (23), polymedication (80.6%) (24), loss of functional independence (from at least one ADL) (25) found in 27.8% of cases in our series, and the level of comorbidities with a high Charlson score, which was associated with the risk of no longer being able to stay at home in patients with falls (2). The proportion of women in our study may appear elevated at 73%, with an average age of 83, even though they constitute a slightly smaller portion, around 65%, of individuals aged 80 and above in France. However, it is well-documented that women at advanced ages face a heightened risk of falls compared to men, which explains the higher representation of women in our study compared to the general population. This observation aligns with findings from other studies, such as one involving a population of 454 individuals referred for clinic assessment in thirteen outpatient falls clinics in Victoria, Australia, where the female proportion was 73% (26). Additionally, a review involving 3.580 subjects investigating the relationship between muscle mass and physical performance showed a female proportion of 70%, which is close to our own findings (11).
In a Taiwanese cross-sectional study of 280 subjects (aged 65-91) (27), 68.9% were women, and as in our study, there was no significant difference between the muscle mass of the two legs of fallers and non-fallers. In a prospective study (28) with a 10-year follow-up of 1041 subjects (50% women aged 63+/-7.5 years), low grip strength was associated with an increased risk of falls and fractures, whereas low muscle mass (appendicular mass adjusted for BMI) seems more appropriate for judging 10-years mortality risk. Our study confirmed that there is indeed a relationship between lower limb muscle mass and grip strength, but this relationship does not appear to be proportional. Several authors have recently insisted on the need to think about the quality of the muscle more than the muscle mass itself (29). Maintaining muscle strength appears to have a more positive impact on functional independence than maintaining muscle mass (11).
In our study, possible sarcopenia existed in 67% and 80% of the patients included in our study, according to the chair lift test and grip test respectively (9). The absence of data on appendicular mass does not allow us to know the exact prevalence of sarcopenia (if we refer to the most recent definition criteria), but the high proportion of subjects with a sedentary lifestyle (89.6% with more than 10 hours/day) suggest that it must be particularly high, more so than in non-dropout OP. In a cross-sectional study of 1.165 subjects living at home, the cut-off point for determining a sedentary lifestyle predictive of sarcopenia was > 120 min/d (TV program) – and < 20 min.day-1 use of digital tools (30). These results are reinforced by a meta-analysis involving over a million people, in which Ku et al found a sedentary lifestyle cut-off at 9 h predictive of all-cause mortality (31). Within this vulnerable population, individuals with reduced muscle strength can be identified, even if they exhibit minimal or no loss of muscle mass. The European criteria for sarcopenia might lead to under-diagnosis in these cases, whereas Asian guidelines (27), which prioritize muscle strength, could facilitate more effective treatment strategies for such individuals (11)
Our study found no correlation between muscle mass and the level of physical activity measured by the number of steps. The literature does not report any data on this subject in OP suffering from falls. The majority of patients had a very low level of physical activity, as evidenced by the average number of steps over 5 days (1313 (507 – 2795)) and sedentary activity time (13.3 ± 2.5 hours). This finding was expected. In a meta-analysis of objectively measured physical activity, sedentary behavior in OP subjects living at home was associated with falls and fear of falling (32). In a prospective longitudinal study, global energy expenditure was measured by actimetry in 870 participants (mean age: 82) for 9 days, and 584 people were followed for an average of 3.4 years (33). The data suggested that physical activities of daily living could contribute to the prevention of disability, even if this activity remained moderate in frail subjects. This lack of correlation between muscle mass and number of steps could be explained in two ways. Firstly, with age, the distribution of muscle fibres changes, with an increase in type I fibres partially compensating for the decrease in type II fibres. This modification may explain a loss of power resulting in a reduction in the number of steps without a parallel reduction in muscle mass (34). Secondly, the use of a sensor enables count recording.These devices have their limits of precision. This depends on the sensor technology (IMU or triaxial accelerometer), the sensitivity of the measurement, and the location of the equipment. In fact, the sensor would be more sensitive if positioned at the hip for slow gaits (35). The armband (SenseWear®) used in this study has been validated for use on the arm (36). It is possible that the number of steps may have been underestimated when recording in slow walking mode, associated with step shortening.
In addition, the wearable sensor quantifies physical activity in terms of energy expenditure. More precisely, this device provides an estimate of total energy expenditure (including basal energy expenditure in the waking state), including voluntary activities such as walking, as well as spontaneous activities of daily living (household chores, DIY, static positions, transitions, locomotion….) (37). In our study, although muscle mass was not correlated with the number of steps, it was correlated with AEE, which therefore seems to be a more interesting parameter to take into account in the frail OP patient.
In some studies, other muscular parameters were taken into account by the authors. Other parameters in OP living at home were assessed : In a cross-sectional study utilizing baseline data from the Health, Aging, and Body Composition study (1997/98) involving 365 well-functioning men and women aged 70 to 79, we observed a correlation between muscle mass and function (walking speed, chair raising) on one hand, and muscle fat infiltration on the other hand (38). In a cross-sectional study (39) in an OP population aged 65-95 with no serious pathology or medication that could have an impact on physical activity, it was found that walking performance (distance covered over 6 min) was linked to fat mass index and muscle mass index in the slowest group, while in the fastest group, it was linked to the chair lift test and, in the intermediate group, to muscle mass index. More recently, Stoever et al (40) showed in men living at home that walking speed and grip test were associated with sarcopenia in subjects over 65.
In our study, other interesting associations were observed. For example, an association was found between lower limb muscle mass and Charlson index, indicating that a high level of comorbidities in a patient with a fall should raise strong suspicions of sarcopenia. Concordantly, in a cross-sectional survey of 168 OP, an association between high Charlson index and sarcopenia was observed (41). Our results show that albumin level (ambulatory patients) was associated with total muscle mass; in a prospective study of 84-year-old middle-aged patients in follow-up rehabilitation care, albumin was not associated with appendicular mass (42). However, it was associated with a risk of infections and pressure sores, which should draw attention to the patient’s nutritional status. Protein and calorie intake recommendations for OP suffering from falls must be increased in the presence of undernutrition, with explanations of the methods of administration and their synergy with physical exercise (2).
In addition, hypovitaminosis D (i.e. a serum 25(OHD) level < 30 ng.ml-1) was found in one out of two patients in our study, and was associated with reduced muscle mass throughout the body and in the lower limbs. Vitamin D receptors are ubiquitously present in body tissues. In various studies, hypovitaminosis D has been associated with multiple chronic diseases and their severity (43) frailty (44) and risk of repeated falls (45). This association has already been reported in previous studies. Visser et al (46) found an association between vitamin D levels < 10 ng.ml-1 and reduced muscle mass using DEXA in a 3-year prospective study. In OP patients who have suffered a fall, vitamin D levels should be measured to identify any deficiency and correct it if it exists (47). The aim is to prevent the consequences of such a deficit on bone and lower limb muscular strength (48) and balance (49). Correcting vitamin D deficiency could therefore help to limit falls, fractures, sarcopenia and loss of functional independence in OP (50).
Our results, like those in the literature, encourage us to take a close look at the physical activity levels of our older patients who fall or are at high risk of falling (2). Resistance training programs, even for subjects with a sedentary profile, have positive effects on muscular strength and motor performance (51). However, to achieve a significant reduction in the incidence of falls, the FICSIT study showed that resistance training must be systematically accompanied by balance training in OP subjects who fall (52). This factor, which is classically modifiable in younger subjects (53) becomes potentially more difficult to enhance in older subjects. For example, it is easier to explain the benefits of physical activity in limiting cardiovascular pathologies than the insidious nature of sarcopenia. A sedentary lifestyle, on the other hand, exposes older people to an increased risk of falls (13) fracture (54) often associated with fear of falling (55). The resulting negative psychological impact contributes to unconscious avoidance attitudes (7) which increase the reduction in physical activity, including the routine activities of daily living (shopping, socializing with neighbors). More recently, Ramsey et al. (56), in a meta-analysis of 27.629 OP who had fallen at home, showed the importance of the fear of falling factor, which is associated with less enthusiasm for physical activity (inverse correlation) and a propensity for a sedentary lifestyle.
Through a personalized approach with the OP, it is important to identify with them the obstacles to physical activity that contribute to their sedentary lifestyle, and to find the facilitators that make it easier for them to get involved.
Within the limits of this study, we retain appendicular muscle mass, which was not measured (our study was designed prior to publication of the EWGSOP definition) (9). This makes it impossible to determine the prevalence of sarcopenia in our population. Other limitations are related to the participant count; initially planned for 200, we recruited 170 individuals. Additionally, technical constraints of measurement tools in older adults during slow walking may lead to an underestimation of step counts by the armband devices. Lastly, there may be a need to reassess outcome thresholds to accommodate the oldest and most vulnerable subjects in our study population, with an average age of 83 years and ranging up to 93 years.
Conclusion
In this study OP seeking consultation for falls or gait disorders, AEE was associated with whole-body and lower-limb muscle mass. Multivariate analyses revealed associations between muscle mass and various factors, including AEE, albumin levels, Charlson index, gender, and vitamin D deficiency. The study sheds light on the original findings in a high-risk older people, emphasizing that despite their frailty and low physical activity, maintaining daily physical activity is linked to better preservation of muscle mass. The study also underscores the prevalence of possible sarcopenia and the importance of assessing factors like hypovitaminosis D and comorbidities in this population. Notably, the study suggests that AEE may be a more meaningful parameter than the number of steps in evaluating physical activity in frail older adults. The findings advocate for a nuanced approach to understanding the complex interplay between muscle mass, physical activity, and various health indicators in this vulnerable demographic.
Key Points
• Loss of muscle mass is correlated with reduced energy expenditure in a population of older adults who experience falls and/or are at high risk of fall.
• Muscle mass was not correlated with the number of daily steps. The potential underestimation of step counts by motion sensors during slow walking, attributed to shortened steps, underscores the importance of standardized quantification of physical activity. This consideration should encompass technical limitations and interpretation biases to ensure accurate comparisons.
• Even within this frail population with low physical activity and at risk of loss of mobility and functional dependence, maintaining regular daily physical activity is associated with better preservation of muscle mass.
• The personalized care in this population who fall and/or at high risk of falling, should aim to provide tailored daily or twice-weekly physical activity.
Acknowledgments: Authors would like to thank all of our participants for volunteering in this study.
Conflict of interest statement: The authors have nothing to disclose. All authors report no conflicts of interest.
Author Contributions: JBG: Substantial contributions to the conception and design of the work; analysis, and interpretation of data. Drafting the work. SM: Contributions to the interpretation of data for the work and contribution to the drafting of the work. MM, MP, PG: Substantial contributions to the acquisition of data for the work. JG: Substantial contributions to the analysis and interpretation of data for the work. CA: Final approval of the version to be published. FP: Revising the work critically for important intellectual content and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Impact Statement: Our study focused on older adults consulting for falls or gait disorders, highlighting associations between muscle mass and various factors such as total energy expenditure (AEE), albumin levels, Charlson index, gender, and vitamin D deficiency. Despite low physical activity levels, maintaining daily activity correlated with better preservation of muscle mass in this population. Notably, the prevalence of sarcopenia was high, emphasizing the importance of assessing factors such as nutrition, hypovitaminosis D, comorbidities, and a sedentary lifestyle. The study suggests that AEE may be a more meaningful parameter than step counts for evaluating physical activity in frail older adults. It underscores the need for comprehensive assessments in this population to identify sarcopenia and potential interventions to prevent falls and maintain functional independence. By highlighting the complex interplay between muscle mass, physical activity, and health indicators, our findings contribute to a better understanding of strategies to prevent falls and improve well-being in vulnerable older adults.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas 2013;75:51–61
2. Montero-Odasso M, van der Velde N, Martin FC, et al. World guidelines for falls pre-vention and management for older adults: a global initiative. Age Ageing 2022;51:afac205
3. Puisieux F, Pollez B, Deplanque D, et al. Successes and setbacks of the falls consulta-tion: report on the first 150 patients. Am J Phys Med Rehabil 2001;80:909–915
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;2012:CD007146
5. Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2004;52:1121–1129
6. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implica-tions for clinical practice and public health. Lancet 2019;394:1365–1375
7. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2019;10:485–500
8. Song S, Geyer H. Predictive neuromechanical simulations indicate why walking perfor-mance declines with ageing. J Physiol 2018;596:1199–1210
9. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601
10. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: A review. Eur J Intern Med 2016;31:3–10
11. Riviati N, Indra B. Relationship between muscle mass and muscle strength with physical performance in older adults: A systematic review. SAGE Open Med 2023;11:20503121231214650
12. Pierre J, Collinet C, Schut P-O, Verdot C. Physical activity and sedentarism among sen-iors in France, and their impact on health. PLoS One 2022;17:e0272785
13. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev 2019;1:CD012424
14. Treacy D, Hassett L, Schurr K, Fairhall NJ, Cameron ID, Sherrington C. Mobility train-ing for increasing mobility and functioning in older people with frailty. Cochrane Database Syst Rev 2022;6:CD010494
15. Ramsey KA, Rojer AGM, D’Andrea L, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in old-er adults: A systematic review and meta-analysis. Ageing Res Rev 2021;67:101266
16. World Health Organization. Recommandations mondiales sur l’activité physique pour la santé. 2010. Organisation mondiale de la santé, Genève
17. Folstein, MF, Folstein, SE, McHugh, PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician – PubMed. https://pubmed.ncbi.nlm.nih.gov/1202204/. Accessed 22 Nov 2023
18. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983;31:721–727
19. Neilson HK, Robson PJ, Friedenreich CM, Csizmadi I. Estimating activity energy ex-penditure: how valid are physical activity questionnaires? Am J Clin Nutr 2008;87:279–291
20. Delbaere K, Hauer K, Lord SR. Evaluation of the incidental and planned activity ques-tionnaire (IPEQ) for older people. Br J Sports Med 2010;44:1029–1034
21. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251
22. Tavassoli N, Guyonnet S, Abellan Van Kan G, et al. Description of 1,108 older patients referred by their physician to the “Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability” at the gerontopole. J Nutr Health Aging 2014;18:457–464
23. Verghese J, Ambrose AF, Lipton RB, Wang C. Neurological gait abnormalities and risk of falls in older adults. J Neurol 2010;257:392–398
24. Dhalwani NN, Fahami R, Sathanapally H, Seidu S, Davies MJ, Khunti K. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open 2017;7:e016358
25. Zhang Q, Zhao X, Liu H, Ding H. Frailty as a predictor of future falls and disability: a four-year follow-up study of Chinese older adults. BMC Geriatr 2020;20:388
26. Hill KD, Moore KJ, Dorevitch MI, Day LM. Effectiveness of falls clinics: an evaluation of outcomes and client adherence to recommended interventions. J Am Geriatr Soc 2008;56:600–608
27. Yang N-P, Hsu N-W, Lin C-H, et al. Relationship between muscle strength and fall epi-sodes among the elderly: the Yilan study, Taiwan. BMC Geriatr 2018;18:90
28. Balogun S, Winzenberg T, Wills K, et al. Prospective Associations of Low Muscle Mass and Function with 10-Year Falls Risk, Incident Fracture and Mortality in Community-Dwelling Older Adults. J Nutr Health Aging 2017;21:843–848
29. Nogueira Paranhos Amorim D, Nascimento DC, Stone W, Alves VP, Moraes CF, Coe-lho Vilaça E Silva KH. Muscle Quality Is Associated with History of Falls in Octogenarians. J Nutr Health Aging 2021;25:120–125
30. de Souza LF, Canever JB, Moreira B de S, Danielewicz AL, de Avelar NCP. Associa-tion Between Fear of Falling and Frailty in Community-Dwelling Older Adults: A Systematic Review. Clin Interv Aging 2022;17:129–140
31. Ku P-W, Steptoe A, Liao Y, Hsueh M-C, Chen L-J. A cut-off of daily sedentary time and all-cause mortality in adults: a meta-regression analysis involving more than 1 million par-ticipants. BMC Med 2018;16:74
32. Ramsey KA, Meskers CGM, Maier AB. Every step counts: synthesising reviews asso-ciating objectively measured physical activity and sedentary behaviour with clinical outcomes in community-dwelling older adults. Lancet Healthy Longev 2021;2:e764–e772
33. Shah RC, Buchman AS, Leurgans S, Boyle PA, Bennett DA. Association of total daily physical activity with disability in community-dwelling older persons: a prospective cohort study. BMC Geriatr 2012;12:63
34. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quan-titative review. Front Physiol 2012;3:260
35. Guediri A, Robin L, Lacroix J, Aubourg T, Vuillerme N, Mandigout S. Comparison of Energy Expenditure Assessed Using Wrist- and Hip-Worn ActiGraph GT3X in Free-Living Conditions in Young and Older Adults. Front Med (Lausanne) 2021;8:696968
36. Andre D, Pelletier R, Farringdon J, et al. The development of the SenseWear® armband, a revolutionary energy assessment device to assess physical activity and lifestyle. BodyMedia Inc 2006
37. Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogene-sis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 2008;294:R699-710
38. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904
39. Marques E, Carvalho J, Soares JMC, Marques F, Mota J. Effects of resistance and mul-ticomponent exercise on lipid profiles of older women. Maturitas 2009;63:84–88
40. Stoever K, Heber A, Eichberg S, Brixius K. Sarcopenia and Predictors of Skeletal Mus-cle Mass in Elderly Men With and Without Obesity. Gerontol Geriatr Med 2017;3:2333721417713637
41. Gong G, Wan W, Zhang X, Liu Y, Liu X, Yin J. Correlation between the Charlson comorbidity index and skeletal muscle mass/physical performance in hospitalized older people potentially suffering from sarcopenia. BMC Geriatr 2019;19:367
42. Bouillanne O, Hay P, Liabaud B, Duché C, Cynober L, Aussel C. Evidence that albumin is not a suitable marker of body composition-related nutritional status in elderly patients. Nutri-tion 2011;27:165–169
43. Beauchet O, Dubost V, Nevers A, et al. [Development of a clinical test of gait in frail elderly by a cognitive approach of locomotion]. Ann Readapt Med Phys 2002;45:123–130
44. Annweiler C, Souberbielle J-C, Schott A-M, de Decker L, Berrut G, Beauchet O. [Vit-amin D in the elderly: 5 points to remember]. Geriatr Psychol Neuropsychiatr Vieil 2011;9:259–267
45. Snijder MB, van Schoor NM, Pluijm SMF, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 2006;91:2980–2985
46. Visser M, Deeg DJH, Lips P, Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–5772
47. Souberbielle J-C, Cormier C, Cavalier E, et al. Vitamin D supplementation in France in patients with or at risk for osteoporosis: recent data and new practices. Joint bone spine 2020;87:25–29
48. Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insuffi-ciency. J Am Geriatr Soc 2010;58:2063–2068
49. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:551–561
50. Nakamura K, Nishiwaki T, Ueno K, Yamamoto M. Serum 25-hydroxyvitamin D levels and activities of daily living in noninstitutionalized elderly Japanese requiring care. J Bone Mi-ner Metab 2005;23:488–494
51. Morganti CM, Nelson ME, Fiatarone MA, et al. Strength improvements with 1 yr of progressive resistance training in older women. Med Sci Sports Exerc 1995;27:906–912
52. Province MA, Hadley EC, Hornbrook MC, et al. The effects of exercise on falls in el-derly patients. A preplanned meta-analysis of the FICSIT Trials. Frailty and Injuries: Coopera-tive Studies of Intervention Techniques. JAMA 1995;273:1341–1347
53. Blain H, Bloch F, Borel L, et al. Activité physique et prévention des chutes chez les per-sonnes âgées. 2015. PhD Thesis, Institut national de la santé et de la recherche médicale (IN-SERM)
54. Levesque M, Ndangang M, Riaudel T, de Decker L, Benichou J, Berrut G. Relationship between body composition and bone mineral density, related to physical activity, in elderly women. Geriatr Psychol Neuropsychiatr Vieil 2016;14:398–405
55. Gaxatte C, Nguyen T, Chourabi F, et al. Fear of falling as seen in the Multidisciplinary falls consultation. Ann Phys Rehabil Med 2011;54:248–258
56. Ramsey KA, Zhou W, Rojer AGM, Reijnierse EM, Maier AB. Associations of objec-tively measured physical activity and sedentary behaviour with fall-related outcomes in older adults: A systematic review. Ann Phys Rehabil Med 2022;65:101571
© The Author(s) 2024