A.M. Wennberg1, S. Ek1, M. Na2
1. Unit of Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden; 2. Department of Nutritional Science, Penn State College of Health and Human Development, University Park, PA, USA
Corresponding Author: Alexandra Wennberg, Nobels väg 13, 171 77 Stockholm, Sweden, Alexandra.wennberg@ki.se
J Frailty Aging 2024;13(3)285-292
Published online April 10, 2024, http://dx.doi.org/10.14283/jfa.2024.21
Abstract
BACKGROUND: Both food insecurity (FI) and vision impairment (VI), which are linked, have been independently associated with frailty and falls.
OBJECTIVES: Understand how FI and VI may together contribute to frailty and fall risk could improve insight into these growing public health challenges.
DESIGN, SETTING, PARTICIPANTS, MEASUREMENTS: This study included 5,963 participants aged 65 and older enrolled in the National Health and Aging Trends Study. Participants were divided into four exposure groups (“No FI or VI,” “FI, no VI,” “VI, no FI,” and “Both”) based on self-report. The Fried Frailty Index and self-reported falls were assessed annually. We used adjusted logistic and Poisson regression models to examine cross-sectional associations and generalized estimating equations to examine longitudinal associations between FI/VI status and falls and frailty outcomes.
RESULTS: Most study participants reported neither FI nor VI (n=5169, 86.7%); however, having both FI and VI (n=57, 1%) was cross-sectionally associated with higher frailty score and higher odds of falling multiple times in the last year. FI and/or VI were longitudinally associated with higher frailty score and increased frailty risk, with the strongest association for Both (RRR=1.29, 95% CI 1.23, 1.58; OR=3.18, 95% CI 1.78, 5.69), and with falling, again highest among those with Both, for one (OR=2.47, 95% CI 1.41, 3.96) and multiple (OR=2.46, 95% CI 1.50, 4.06) falls in the last year.
CONCLUSION: Clinical and public health interventions could address the intersection of FI and VI with the aim of ameliorating the impact of these risk factors and health outcomes.
Key words: Longitudinal, nationally-representative, modifiable risk factors.
Introduction
Falls and frailty are becoming more common as the older adult population increases, and both are associated with loss of independence, institutionalization, and mortality. The driving mechanisms for falls and frailty are multifactorial (1, 2), but many risk factors are modifiable and can be addressed to reduce the risk of falls and frailty occurring and their associated long term consequences. Vision impairment (VI) and food insecurity (FI) are two modifiable conditions that are also on the rise among older adults and, although perhaps seemingly unrelated, new evidence suggests they are connected and increase risk of falls and frailty in older adults.
VI is common among older adults and increases with increasing age, with over 20% of individuals over age 80 experiencing vision impairment or blindness in the U.S. (3). It has long been associated with increased risk of falling (4, 5) and, more recently, with frailty (6). FI is also on the rise among older adults in the U.S., with nearly 15% of older adults who live alone experiencing FI and older adults making up nearly 16% of Supplemental Nutrition Assistance Program (SNAP) participants (7). The increasing food insecurity among older adults makes the associated health outcomes, including the risk of falling (8) and frailty (9, 10), even more of a concern, though this evidence comes from cross-sectional study designs.
Emerging research suggests that FI and VI are linked. In a recent cross-sectional study, FI was associated with a 1.85 higher odds of self-reported VI and 2.70 times higher odds of objectively measured VI, independent of age, gender, race, socioeconomic status, substance use, body mass index, and physical activity (11). FI via poor diet is associated with many conditions that either cause or lead to VI (e.g., age-related macular degeneration, diabetes) (11, 12). However, it is also possible that FI may be a flag of poorer resource access overall and be related to limited healthcare access and chronic stress, which could impact vision. Further, it has been shown that poorer resource access is also associated with barriers to VI diagnosis and eye care (13-15), so this association is likely underestimated. Alternatively, it has also been shown that VI is associated with 1.46 times higher odds of FI, in comparison to other forms of disability, which may be related to challenges with decreased earnings and costs for assistive and adaptive equipment (16). Older adults with VI may be particularly vulnerable, because of the added barriers in completing activities of daily living, such as acquiring and preparing food (17). These layered risk factors, which impact comorbid status and physical function, may compound the risk for falls and frailty among older adults. Therefore, in this study, we aimed to examine the co-occurrence of FI and VI among older adults and how they are, separately and together, associated with both falls and frailty cross-sectionally and, importantly, longitudinally.
Methods
Data and Participants
This study used data from the National Health and Aging Trends Study (NHATS), which is a nationally representative survey of Medicare beneficiaries living in the United States aged 65 years and older (18). NHATS used a complex survey sampling design and oversampled for non-Hispanic Black individuals and those aged 90 and older. In 2011 (Wave 1), NHATS enrolled 8,245 participants and followed them annually to gather data on socioeconomic status, health conditions, social aspects, physical environment, and physical and cognitive functioning. Participants complete annual in-person standardized assessments with a trained interviewer in their own home. In the current analyses, we included participants with complete data for FI experience and VI at Wave 2 (2012) and frailty or falls assessments at Wave 2 and at least one subsequent wave. Participants were followed for a maximum of 8 years (2012-2019), corresponding to Waves 2-9. A total of 5,963 participants were included in the present study. NHATS is funded by the National Institute on Aging (U01AG032947) and conducted by the Johns Hopkins Bloomberg School of Public Health. The NHATS and NSOC were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board and all participants provided informed consent.
Measures
Food insecurity experience and classification
Participants were asked about their FI experience due to financial, social, and functional limitations. Participants were asked about whether they had experienced 1) “going without groceries”, 2) “going without hot meals”, 3) “going without eating due to lack of ability”, 4) “going without eating because of lack of social support to do so”, and 5) “skipping meals due to financial constraints” in the last 30 days. Using methods with strong construct validity developed by Tucher et al. and detailed elsewhere (19, 20), the number of affirmative answers were summed to give an indicator of FI. Participants with a sum of 0 were considered food sufficient and those with a score of ≥1 were considered to have FI.
Vision Impairment
VI was based on self-report of any of the following: blindness, unable to see well enough to recognize someone across the street, unable to see well enough to watch the television across the room, or unable to see well enough to read newspaper print.
Exposure Group Categorization
We created four groups based on the FI and VI variables: 0=no food insecurity or vision impairment (No FI or VI), 1=food insecure, no vision impairment (FI, no VI), 2=vision impairment, not food insecure (VI no FI), and 3=both food insecurity and vision impairment (Both FI and VI).
Frailty
Frailty was assessed annually with the validated Fried Frailty Index (21) using the annually collected data on slowness, weakness, weight loss, low physical activity, and exhaustion (22). Slowness was assessed with walking speed using the first of two tests of a 3m walk and categorized at or below the 20th percentile by sex and height. Weakness was assessed with grip strength, using the maximum of two tests using a handheld dynamometer and categorized at or below the 20th percentile by sex and body mass index (BMI). Weight loss was categorized as participants who reported unintentional weight loss of ≥10 pounds in the last year. Low physical activity was categorized as reporting never walking for exercise or never engaging in vigorous activities. Finally, exhaustion was categorized as self-reported low energy or ease of exhaustion that limited activities. A frailty sum score (0-5) was calculated by summing the individual items. To create a binary variable, participants with a score ≥3 were considered frail and those with a score 0-2 were considered non-frail (22).
Falls
Falls were assessed at the annual interview via self-report. Participants were asked about falling, including any fall, slip, or trip in which you lose your balance and land of the floor or ground at a lower level.” In this study, we used the variables where participants were asked if they had fallen “in the last 12 months” and, if they had fallen in the last 12 months, were asked if they had “fallen down more than one time.” Both variables were categorized as dichotomous variables for analyses.
Covariates
Baseline covariates collected at Wave 2 were included in cross-sectional and longitudinal analyses. Sociodemographic covariates included: sex (woman or man), age group (65-69, 70-74, 75-79, 89-84, 85-89, and ≥90), race/ethnicity (White, non-Hispanic Black, other), education (<high school, high school, >high school), total income (divided into quintiles), marital status (married/living with a partner or not), and BMI (kg/m2, based on self-reported height and weight). We additionally created a comorbidity count that included the following self-reported health conditions: heart attack, heart disease, stroke, cancer, osteoporosis, lung disease, and arthritis. We separately adjusted for hypertension, diabetes, and dementia. Finally, overall health assessed by asking participants about their general overall health (1=excellent, 2=very good, 3=good, 4=fair, 5=poor), and anxiety and depression symptoms, assessed with the 2-item Generalized Anxiety Disorder Scale (23) and 2-item Patient Health Questionnaire (24) were also included as covariates.
Statistical analysis
We first compared participant characteristics by the four groups defined by FI and VI status at baseline (i.e., “No FI or VI,” “FI, no VI,” “VI, no FI,” and “Both”). We used baseline FI and VI status instead of time-varying FI and VI status, because we found that it was uncommon for people to change FI/VI groups over time and groups remained largely the same across Waves. We used regression models to examine the cross-sectional association between FI and VI status and frailty and fall outcomes at Wave 2. All binary outcomes (frailty, fall in the last year, and multiple falls in the last year) were modeled with logistic regression. Frailty score sum, due to its discreet probability, right-skewed distribution, was modeled with Poisson regression and coefficients presented as incidence rate ratios (IRR). In longitudinal analyses, we again used logistic and Poisson generalized estimating equation models to examine frailty and fall outcomes. Time was modeled as a continuous variable and an interaction term between time and FI/VI group was created to examine the association over time. Postestimation allowed us to present these coefficients as odds ratios (OR) and relative risk ratios (RRR). Margins plots were created to visualize the trends over time. In both cross-sectional and longitudinal analyses, model 1 was adjusted for age and sex. In model 2, for frailty outcomes, we adjusted for age, sex, race, education, income, BMI, overall health, hypertension, diabetes, dementia, comorbidity count, and depression and anxiety symptoms. For fall outcomes, model 2 was adjusted for age, sex, race, education, income, BMI, overall health, dementia, comorbidity count, and depression and anxiety symptoms.
Results
In our sample, most participants (n= 5169, 86.7%) reported having neither FI nor VI, while 3.1% (n=182) reported FI without VI, 9.3% (n=555) reported VI without FI, and 1.0% (n=57) reported both (Table 1). Weighted baseline characteristics, using survey weights to represent a population of 33,346,365, are presented in Supplementary Table 1. Women and older people were more represented in the groups with FI and VI. White participants were most common in the No FI or VI group, Black participants most common in the VI, no FI group, and participants with another race were most common in the Both FI and VI group. Participants with lower educational attainment and income were more represented in the groups with some FI and/or VI. Similarly, participants in the groups with FI and/or VI were more likely to report having other health conditions.
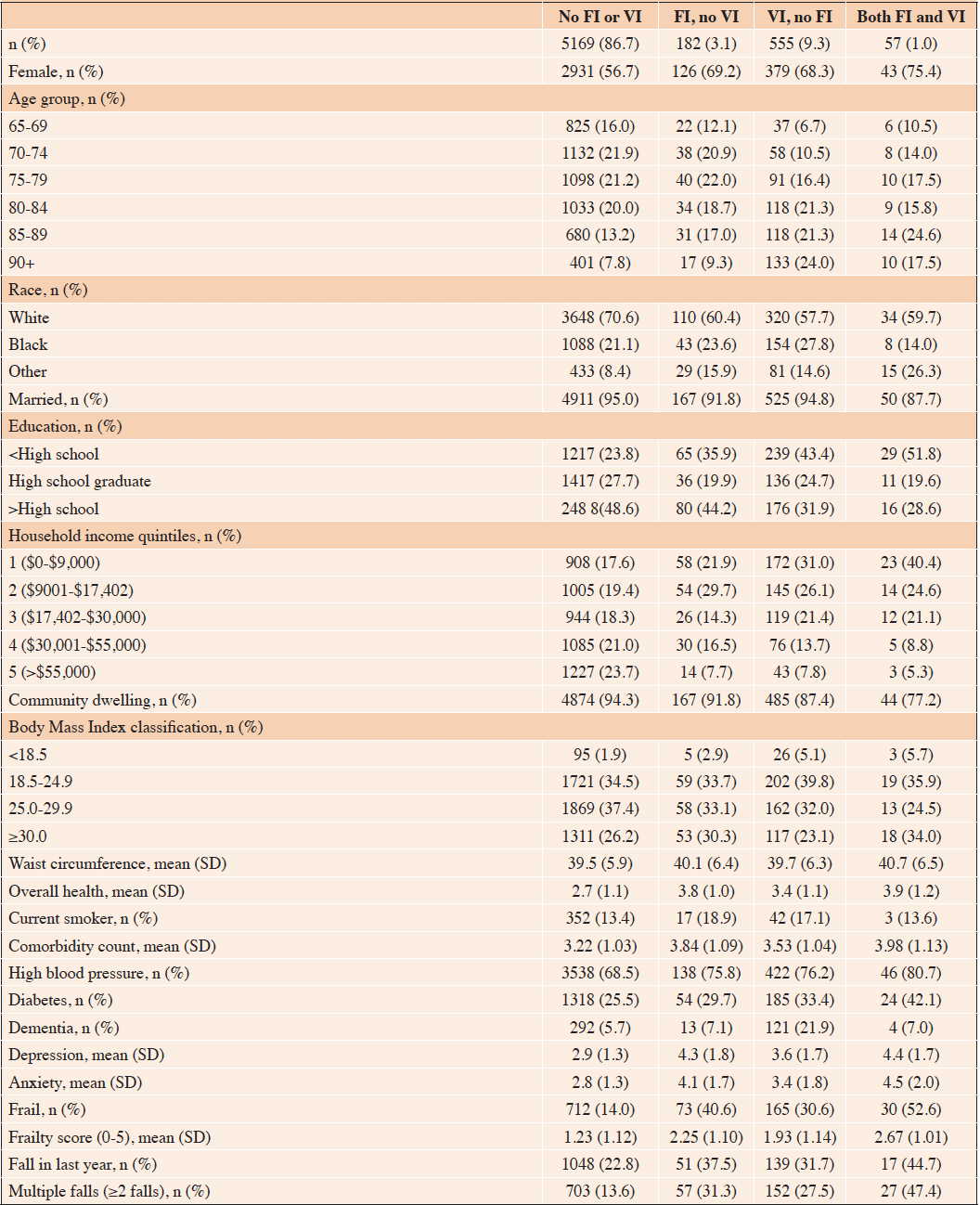
Table 1. Characteristics by food insecurity (FI) and vision impairment (VI) status at baseline, n(%) or mean (SD)
FI, food insecurity; VI, vision impairment; Comorbidity count included heart attack, heart disease, stroke, cancer, osteoporosis, lung disease, and arthritis; Overall health self-rated on a scale of 1-5, higher score indicates worse health; Depression and anxiety on a scale of 2-8
In cross-sectional analyses, in the fully adjusted model, compared to those with no FI or VI, those in the FI, no VI and VI, no FI groups did not have higher incidence rate ratios for frailty score sum, though those in the Both FI and VI group did (IRR=1.20, 95% CI 1.05, 1.37) (Table 2). Similarly, those with FI, no VI and with VI, no FI did not have higher odds of being frail, while those with both FI and VI had 3.03 (95% CI 1.47, 6.23) higher odds of being frail. When examining falls, FI and/or VI were not associated with higher odds of falling in the last year; however, having both FI and VI was associated with higher odds of multiple falls in the last year (OR=2.92, 95% CI 1.41, 6.08).
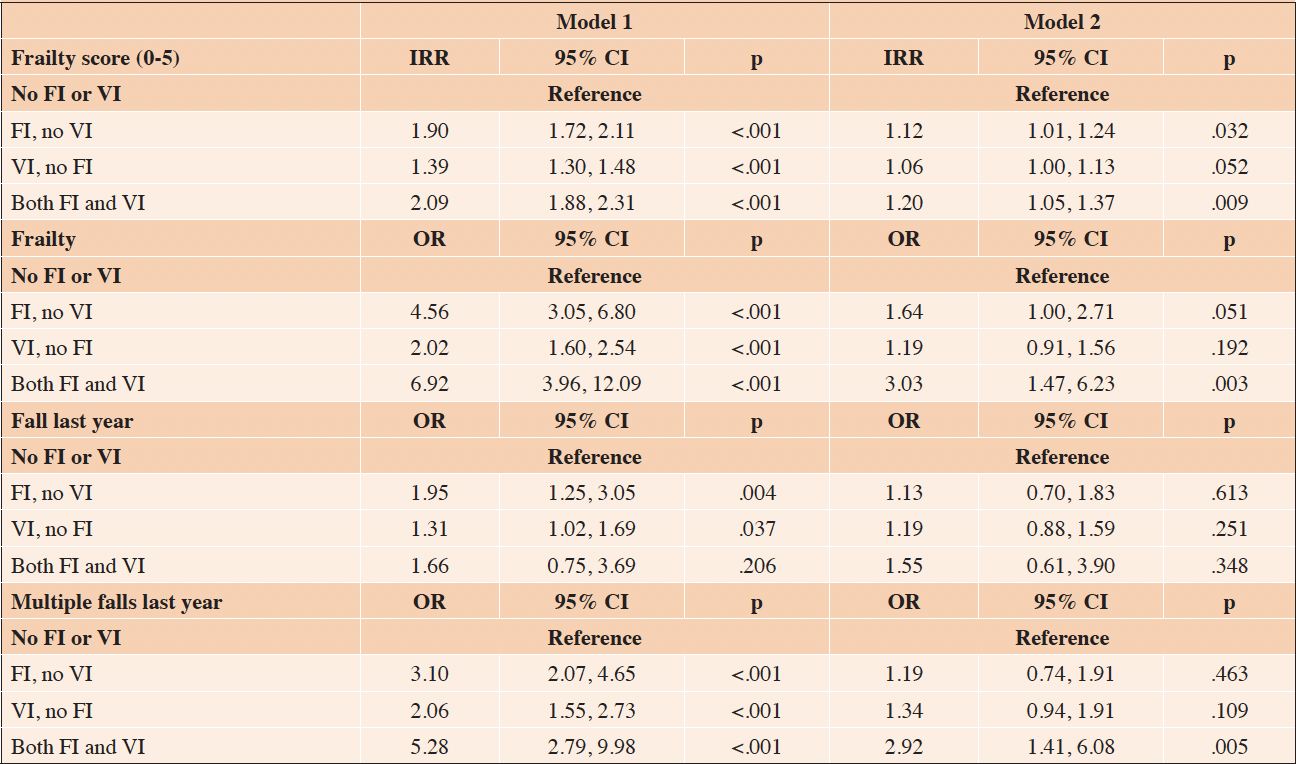
Table 2. Logistic and Poisson regression analyses modeling the cross-sectional association between food insecurity (FI) and vision impairment (VI) status and frailty and fall outcomes, odds ratio (OR) or incidence rate ratio (IRR)
Model 1 adjusted for age and sex. For frailty outcomes, Model 2 adjusted for age, sex, race, education, income, BMI, overall health, hypertension, diabetes, dementia, comorbidity count, and depression and anxiety symptoms. For fall outcomes, Model 2 adjusted for age, sex, race, education, income, BMI, overall health, dementia, comorbidity count, and depression and anxiety symptoms.
In the longitudinal analysis, FI, no VI at baseline was associated with higher frailty score over time (RRR=1.18, 95% CI 1.10, 1.27), as was VI, no FI (IRR=1.15, 95% CI 1.09, 1.22) and Both (IRR=1.39, 95% CI 1.23, 1.58) (Table 3, Figure 1). Similarly, FI, no VI was associated with higher odds of frailty over time (OR=1.63, 95% CI 1.24, 2.13), as was VI, no FI (OR=1.47, 95% CI 1.15, 1.87), and there appeared to be an additive relationship for Both (OR=3.18, 95% CI 1.78, 5.69) (Table 3, Supplementary Figure 1). For fall outcomes, those with FI, no VI (OR=1.63, 95% CI 1.24, 2.13) and VI, no FI (OR=1.47, 95% CI 1.15, 1.87) had higher odds of falling in the last year over time, as did those with both FI and VI (OR=2.37, 95% CI 1.41, 3.96) (Table 3, Figure 2). Finally, FI, no VI (OR=1.65, 95% CI 1.26, 2.15) and VI, no FI (OR=1.52, 95% CI 1.19, 1.93) were both associated with multiple falls in the last year, and both FI and VI showed again an additive relationship (OR=2.46, 95% CI 1.50, 4.06) (Table 3, Supplementary Figure 2). In sensitivity analyses, we explored whether excluding those who were frail at baseline changed the results but found that the RRR did not substantially change, and the trends remained the same across groups (results not shown).
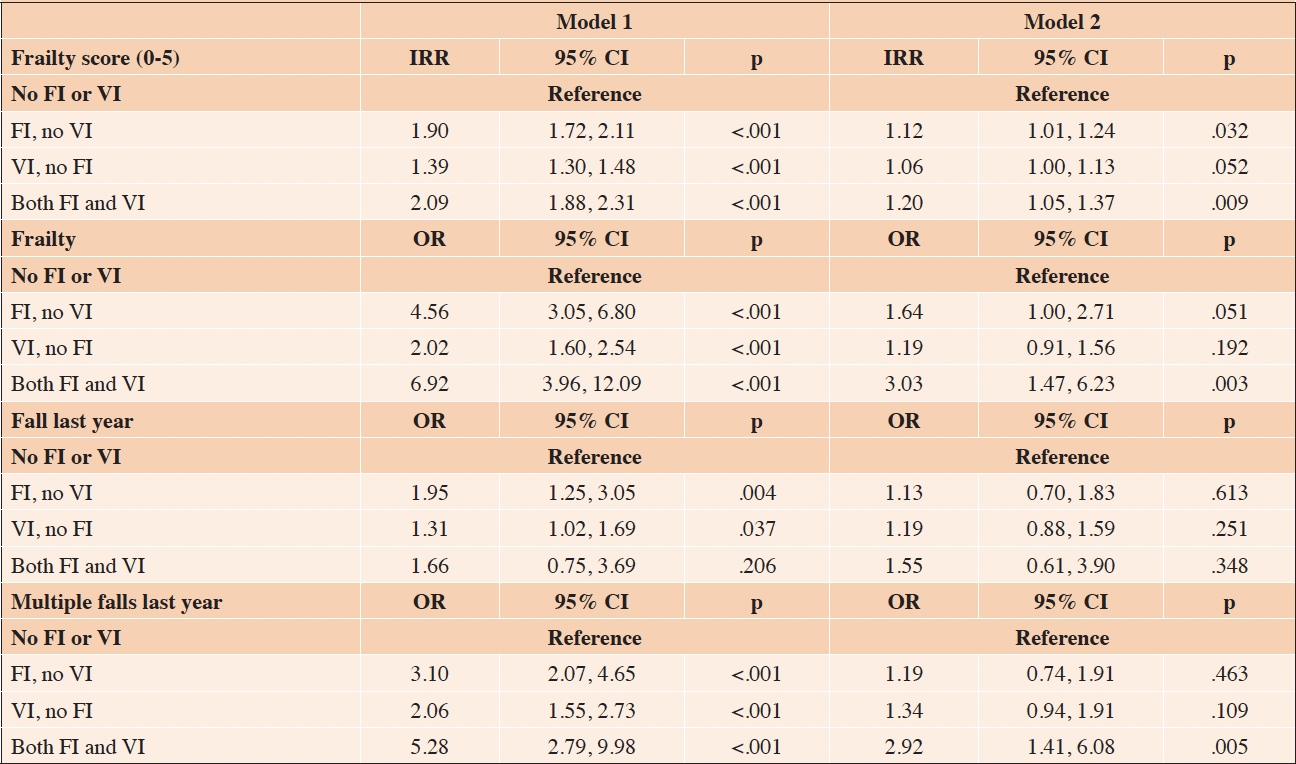
Table 3. Generalized estimating equations modeling the longitudinal association between food insecurity (FI) and vision impairment (VI) status and frailty and fall outcomes over time, relative risk ratios (RRR) and odds ratios for interaction terms (OR)
Model 1 adjusted for age and sex. For frailty outcomes, Model 2 adjusted for age, sex, race, education, income, BMI, overall health, hyper-tension, diabetes, dementia, comorbidity count, and depression and anxiety symptoms. For fall outcomes, Model 2 adjusted for age, sex, race, education, income, BMI, overall health, dementia, comorbidity count, and depression and anxiety symptoms.
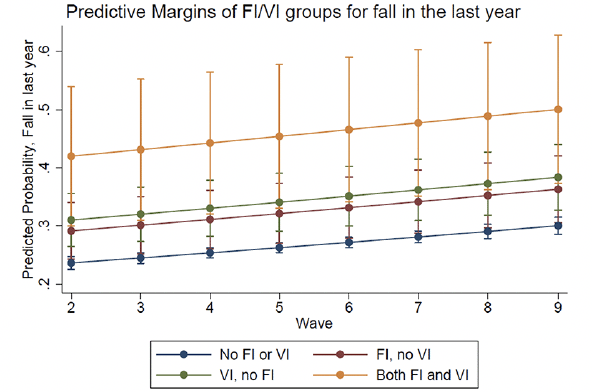
Figure 1. Generalized estimating equation post-hoc predictive margins prediction for frailty score (0-5) over time by food insecurity (FI) and vision impairment (VI) status
Discussion
This study found that both FI and VI were associated with both frailty and risk of falls and that having both FI and VI was associated with even greater risk, independent of demographic, SES, and health factors. The cross-sectional evidence was less robust than the longitudinal evidence showing that having FI and/or VI at baseline was associated with greater risk of both outcomes over the 8 years of follow-up. While both FI and VI were each associated with frailty and falls, there was a consistently slightly stronger association between FI and each outcome. Strikingly, the differences between groups remained much the same over time, suggesting that the risk difference at baseline is maintained longitudinally. This perhaps reflects a persistent and proportional impact of FI/VI status on risk of frailty and falls.
It is unclear exactly how FI is associated with frailty and falling. Some studies have suggested that the stress associated with having FI, compounded by challenges related to lower socioeconomic status and associated disadvantages, could be a driving factor linking FI and frailty and falls (25, 26). However, other studies have suggested that the poor nutritional status among older adults with FI could be the link. Nutritional status is critical for determining health in older adults and it can be both a consequence of underlying conditions and a risk factor for age-related health conditions (27, 28). Although nutritional status is associated with poorer physical performance and several health-related outcomes (29, 30), the multifactorial mechanistic (e.g., sarcopenia, impaired mobility) pathway has yet to be fully elucidated (31). A recent meta-analysis found that older adults who were malnourished or at risk of being malnourished had a 1.45 times higher risk of falling than those who were well-nourished (32). Nutritional status has also been linked to frailty, which may in turn increase risk of falls (29). Poor nutrition can lead to decrease in muscle mass, fat mass, and physical performance, which can lead to sarcopenia and frailty. Overall, several factors, inclusive of multimorbidity and chronic conditions (e.g., VI), can impact the link between poor nutrition and risk of frailty and falls via multiple pathways.
Separately, VI has been linked with falls and frailty among older adults, as well. The higher risk of falls in older adults with VI has been well documented. Older adults with VI have double the risk of falling compared to older adults without VI (33). Another study found that 46.7% of older adults with self-reported severe VI or blindness fell in the last year, compared to only 27.7% of older adults without VI or blindness (34). Similarly, a recent study using NHATS data, which assessed VI using the same methods as the present study, showed that older adults VI are twice as likely to have fallen in the last year as compared to those without VI (27.6% vs 13.2%) (4). The most common VI-causing conditions in older adults (e.g., presbyopia, cataract, glaucoma, macular degeneration) can contribute to risk of falling in a myriad of ways, including impaired depth perception, blurred vision, and limited recognition of objects in the visual field (35). Given these impairments, older adults with VI often have a fear of falling (4) or navigating their daily lives (35), so often reduce their daily activities and are less active. This is proposed as one of the pathways linking VI and higher risk of frailty, as it leads to overall decline in physical functioning and less physical activity (36). However, the link between VI and frailty is understudied by comparison to the falls. In a recent longitudinal study among community-dwelling older adults, self-reported VI was associated with a two-times higher risk of developing frailty, as defined by Fried Frailty Index criteria (37). In a similar study among community-dwelling older adults, those with VI had over three times increased risk of frailty in cross-sectional and longitudinal analysis (6). The study suggested that factors such as social isolation and shared pathological pathways (e.g., inflammation) (38, 39) might also contribute to the link between VI and frailty. Another hypothesis is that VI is associated with several comorbidities (e.g., CVD, diabetes), that are also associated with frailty (37), and this may also be important when considering the interaction between FI and VI.
Research on the relationship between FI and VI is emerging and the relationship is likely bidirectional. In one direction, poor nutrition from being food insecure has been associated with multiple conditions that cause VI, particularly among older adults, including hypertension, hypercholesteremia, diabetes, diabetic retinopathy, cataract, and age-related macular degeneration (11). Further, this can create a positive feedback loop, as recent research has shown that FI is associated with missing recommended eye exams among adults with diabetes (40). In the other direction, adults with VI may have a poorer diet due to difficulties in activities of daily living and acquiring and preparing food, including absence of facilities and assistance (41). Challenges in activities of daily living are faced by older adults without VI, so those with VI face even greater barriers to a nutritious diet. It has also been suggested that long term disability, such as VI, is strongly associated with FI, because perhaps the decreased earning potential and reduced access to nutritious food and healthcare over time negatively impacts health in several ways (16). Indeed, a recent study in over 60,000 US adults living below the 150% Federal Poverty Level it was found that adults with VI, of whom 25% were 65 or older, had double the odds of being food insecure, and there was a dose-response relationship whereby VI more strongly predicted more severe FI (17). The cyclical feedback loop between FI and VI creates a higher risk environment for poor health outcomes, and this is amplified among older adults. Addressing FI in older adults with disabilities, like VI, can mitigate risk of outcomes like falls and frailty. Further, providing older adults with VI with the adequate facilities and assistance needed to acquire and prepare nutritious food is also necessary to mitigate these outcomes.
The study’s limitations must be considered when interpreting the results. First, although we had a relatively large sample size (n=5,963), the number of participants with both FI and VI was relatively small. This may have limited our power, and the findings may have been stronger with a larger both FI and VI group. A larger sample size would have also allowed for exploration of effect modifiers (e.g., gender, race), which may impact proportionality of risk between groups over time. Similarly, because FI and VI were self-reported in this study, there is the possibility for misclassification, though, particularly for FI, this would likely be in the direction of underreporting due to social desirability, and thus our findings may have attenuated towards the null. It may also be that falls outcomes, particularly for a single fall in the last year, is misclassified due to recall; however, the measure of multiple falls in the last year is likely more reliable. Finally, we chose to restrict follow-up from 2011-2019, because in-person NHATS exams were not conducted during the COVID-19 pandemic. However, at the same time, the COVID-19 pandemic increased FI among older adults (42). Therefore, separate studies, specifically investigating the potential increase in health conditions among older adults that would be associated with increased FI during the pandemic and whether these trends have started to reverse since are needed.
In conclusion, FI and VI were longitudinally associated with frailty and falling in this representative sample of older adults in the U.S. Recent findings show that FI and VI are connected and the present findings suggest that older adults with both are more vulnerable regarding these detrimental age-related outcomes. These results emphasize the need for integrating support across clinical and social services to improve the health of older adults, particularly those who are most exposed to these modifiable risk factors. Strategies for mitigating the consequences of FI and VI include screening for FI in primary and vision care settings and screening for VI in primary care settings, even using telemedicine as another tool to reach those at risk (12, 43), as research shows that preserving, and possibly improving, visual function may alleviate FI (11). However, future studies are warranted to develop targeted interventions.
Acknowledgements: none.
Funding: None reported. Open access funding provided by Karolinska Institute.
Conflict of Interest: AMW receives funding from Janssen Pharmaceutica NV for an unrelated project.
Ethical Standards: NHATS is funded by the National Institute on Aging (U01AG032947) and conducted by the Johns Hopkins Bloomberg School of Public Health. The NHATS and NSOC were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board and all participants provided informed consent. All procedures performed in the study were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51-61.
2. Peng Y, Zhong GC, Zhou X, Guan L, Zhou L. Frailty and risks of all-cause and cause-specific death in community-dwelling adults: a systematic review and meta-analysis. BMC Geriatr. 2022;22(1):725.
3. Flaxman AD, Wittenborn JS, Robalik T, Gulia R, Gerzoff RB, Lundeen EA, et al. Prevalence of Visual Acuity Loss or Blindness in the US: A Bayesian Meta-analysis. JAMA Ophthalmol. 2021;139(7):717-23.
4. Ehrlich JR, Hassan SE, Stagg BC. Prevalence of Falls and Fall-Related Outcomes in Older Adults with Self-Reported Vision Impairment. J Am Geriatr Soc. 2019;67(2):239-45.
5. Li Y, Hou L, Zhao H, Xie R, Yi Y, Ding X. Risk factors for falls among community-dwelling older adults: A systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1019094.
6. Swenor BK, Lee MJ, Tian J, Varadaraj V, Bandeen-Roche K. Visual Impairment and Frailty: Examining an Understudied Relationship. J Gerontol A Biol Sci Med Sci. 2020;75(3):596-602.
7. US Department of Agriculture. Food Security and Nutrition Assistance. US Department of Agriculture, Economic Research Service; 2021.
8. Mosen DM, Banegas MP, Friedman N, Shuster E, Brooks N. Food Insecurity Associated with Self-Reported Falls Among Medicare Advantage Members. Popul Health Manag. 2019;22(6):536-9.
9. Muhammad T, Saravanakumar P, Sharma A, Srivastava S, Irshad CV. Association of food insecurity with physical frailty among older adults: study based on LASI, 2017-18. Arch Gerontol Geriatr. 2022;103:104762.
10. Pérez-Zepeda MU, Castrejón-Pérez RC, Wynne-Bannister E, García-Peña C. Frailty and food insecurity in older adults. Public Health Nutr. 2016;19(15):2844-9.
11. Kolli A, Mozaffarian RS, Kenney EL. Food Insecurity and Vision Impairment Among Adults Age 50 and Older in the United States. Am J Ophthalmol. 2022;236:69-78.
12. Gundersen C, Ziliak JP. Food Insecurity And Health Outcomes. Health Aff (Millwood). 2015;34(11):1830-9.
13. Atta S, Zaheer HA, Clinger O, Liu PJ, Waxman EL, McGinnis-Thomas D, et al. Characteristics associated with barriers to eye care: A cross-sectional survey at a free vision screening event. Ophthalmic Res. 2022.
14. Goyal A, Richards C, Patel V, Syeda S, Guest JM, Freedman RL, et al. The Vision Detroit Project: Visual Burden, Barriers, and Access to Eye Care in an Urban Setting. Ophthalmic Epidemiol. 2022;29(1):13-24.
15. Moumneh K, Centeno Gavica J, Centeno Gavica MS, Terrell M, Lomiguen CM. The Role of Barriers and Community-Based Education in Compliance to Regular Eye Exams in New York City’s Harlem Community. Cureus. 2020;12(10):e10875.
16. Coleman-Jensen A, Nord M. Food insecurity among households with working-age adults with disabilities. U.S. Department of Agriculture, Economic Research Service; 2013. Contract No.: ERR-144.
17. Kumar P, Brinson J, Wang J, Samuel L, Swenor BK, Scott AW, et al. Self-Reported Vision Impairment and Food Insecurity in the US: National Health Interview Survey, 2011-2018. Ophthalmic Epidemiol. 2023;30(5):468-76.
18. Montaquila J, Freedman VA, Edwards B, Kasper JD. National Health and Aging Trends Study Round 1 Sample Design and Selection: NHATS Technical Paper #1. Johns Hopkins University School of Public Health; 2012.
19. Na M, Dou N, Brown MJ, Chen-Edinboro LP, Anderson LR, Wennberg A. Food Insufficiency, Supplemental Nutrition Assistance Program (SNAP) Status, and 9-Year Trajectory of Cognitive Function in Older Adults: The Longitudinal National Health and Aging Trends Study, 2012-2020. J Nutr. 2023;153(1):312-21.
20. Tucher EL, Keeney T, Cohen AJ, Thomas KS. Conceptualizing Food Insecurity Among Older Adults: Development of a Summary Indicator in the National Health and Aging Trends Study. J Gerontol B Psychol Sci Soc Sci. 2021;76(10):2063-72.
21. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56.
22. Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-34.
23. Wild B, Eckl A, Herzog W, Niehoff D, Lechner S, Maatouk I, et al. Assessing generalized anxiety disorder in elderly people using the GAD-7 and GAD-2 scales: Results of a validation study. American Journal of Geriatric Psychiatry. 2013;22(10):1029-38.
24. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-13.
25. Arenas DJ, Thomas A, Wang J, DeLisser HM. A Systematic Review and Meta-analysis of Depression, Anxiety, and Sleep Disorders in US Adults with Food Insecurity. J Gen Intern Med. 2019;34(12):2874-82.
26. Laraia BA, Siega-Riz AM, Gundersen C, Dole N. Psychosocial factors and socioeconomic indicators are associated with household food insecurity among pregnant women. J Nutr. 2006;136(1):177-82.
27. Sergi G, Perissinotto E, Pisent C, Buja A, Maggi S, Coin A, et al. An adequate threshold for body mass index to detect underweight condition in elderly persons: the Italian Longitudinal Study on Aging (ILSA). J Gerontol A Biol Sci Med Sci. 2005;60(7):866-71.
28. Vellas BJ, Hunt WC, Romero LJ, Koehler KM, Baumgartner RN, Garry PJ. Changes in nutritional status and patterns of morbidity among free-living elderly persons: a 10-year longitudinal study. Nutrition. 1997;13(6):515-9.
29. Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29(1):43-8.
30. Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875-90.
31. Vandewoude MF, Alish CJ, Sauer AC, Hegazi RA. Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? J Aging Res. 2012;2012:651570.
32. Trevisan C, Crippa A, Ek S, Welmer AK, Sergi G, Maggi S, et al. Nutritional Status, Body Mass Index, and the Risk of Falls in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2019;20(5):569-82.e7.
33. Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18(2):141-58.
34. Crews JE, Chou CF, Stevens JA, Saaddine JB. Falls Among Persons Aged ≥65 Years With and Without Severe Vision Impairment – United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(17):433-7.
35. Reed-Jones RJ, Solis GR, Lawson KA, Loya AM, Cude-Islas D, Berger CS. Vision and falls: a multidisciplinary review of the contributions of visual impairment to falls among older adults. Maturitas. 2013;75(1):22-8.
36. van Landingham SW, Willis JR, Vitale S, Ramulu PY. Visual field loss and accelerometer-measured physical activity in the United States. Ophthalmology. 2012;119(12):2486-92.
37. Liljas AEM, Carvalho LA, Papachristou E, De Oliveira C, Wannamethee SG, Ramsay SE, et al. Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: findings from a 4-year follow-up study. J Epidemiol Community Health. 2017;71(11):1053-8.
38. DeMatteis J, Freedman V, Kasper JD. National Health and Aging Trends Study (NHATS) Round 5 Sample Design and Selection NHATS Technical Paper #16. Baltimore, MD: Johns Hopkins University Bloomberg School of Public Health; 2016.
39. Freedman VA, Kasper JD, Spillman BC. Successful Aging Through Successful Accommodation With Assistive Devices. J Gerontol B Psychol Sci Soc Sci. 2017;72(2):300-9.
40. Gibson DM. Food Insecurity, Eye Care Receipt, and Diabetic Retinopathy Among US Adults with Diabetes: Implications for Primary Care. J Gen Intern Med. 2019;34(9):1700-2.
41. Kostyra E, Żakowska-Biemans S, Śniegocka K, Piotrowska A. Food shopping, sensory determinants of food choice and meal preparation by visually impaired people. Obstacles and expectations in daily food experiences. Appetite. 2017;113:14-22.
42. Ankuda CK, Fogel J, Kelley AS, Byhoff E. Patterns of Material Hardship and Food Insecurity Among Older Adults During the COVID-19 Pandemic. J Gen Intern Med. 2021;36(11):3639-41.
43. Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The Current State of Teleophthalmology in the United States. Ophthalmology. 2017;124(12):1729-34.
© The Author(s) 2024
