Z.-J. Chen1, W.-H. Lu2,3, L.-C. Meng4, W.-F. Chao5, H.-H. Tung6,7, F.-Y. Hsiao4,5,8, L.-K. Chen7,9,10
1. Department of Allied Health Education and Digital Learning, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan; 2. Gerontopole of Toulouse, Institute of Ageing, Toulouse University Hospital (CHU Toulouse), Toulouse, France; 3. Maintain Aging Research team, Centre d’Epidémiologie et de Recherche en santé des POPulations (CERPOP), Inserm, Université Paul Sabatier, Toulouse, France; 4. Graduate Institute of Clinical Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan; 5. School of Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan; 6. Department of Nursing, National Yang Ming Chiao Tung University, Taipei, Taiwan; 7. Center for Healthy Longevity and Aging Sciences, National Yang Ming Chiao Tung University, Taipei, Taiwan; 8. Department of Pharmacy, National Taiwan University Hospital, Taipei, Taiwan; 9. Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, Taipei, Taiwan; 10. Taipei Municipal Gan-Dau Hospital, Taipei, Taiwan
Corresponding Author: Liang-Kung Chen, Professor, Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, No. 201, Sec 2, Shih-Pai Road, Taipei 11217, Taiwan, Tel: +886-2-28757830; Fax: +886-2-28757711, E-mail: lkchen2@vghtpe.gov.tw; Fei-Yuan Hsiao, Ph.D., Professor and director, Graduate Institute of Clinical Pharmacy, College of Medicine,National Taiwan University, Taipei, Taiwan, 33, Linsen S. Rd, Taipei 10050, Taiwan, Tel: +886-2-33668787, E-mail: fyshsiao@ntu.edu.tw
J Frailty Aging 2024;13(4)541-551
Published online September 10, 2024, http://dx.doi.org/10.14283/jfa.2024.66
Abstract
IMPORTANCE: Intrinsic capacity (IC), defined by the World Health Organization’s Integrated Care for Older People (ICOPE) framework, is crucial for promoting healthy aging. Understanding the associations between IC impairments and age-related biomarkers can provide insights into the underlying pathophysiological mechanisms and potential interventions.
OBJECTIVE: To investigate the associations between IC impairments (ICOPE step 1 and step 2, respectively) and aging-related biomarkers, including inflammatory and cardiometabolic markers, in community-dwelling middle-aged and older adults.
DESIGN, SETTING, AND PARTICIPANTS: Cross-sectional analysis of data from 755 participants (aged 50-64 years, n=212; 65-74 years, n=357; ≥75 years, n=186) enrolled in the Gan-Dau Healthy Longevity Plan, a community-based survey in Taipei City, Taiwan, from 2022.
EXPOSURES: IC impairments assessed by ICOPE Step 1 (screening) and Step 2 (in-depth assessment) across six domains: locomotion, vitality, vision, hearing, cognition, and psychological well-being.
MAIN OUTCOMES AND MEASURES: Levels of inflammatory biomarkers (albumin, white blood cell count, neutrophils, lymphocytes, monocytes, neutrophil-to-lymphocyte ratio [NLR], lymphocyte-to-monocyte ratio [LMR], platelet-to-lymphocyte ratio [PLR]) and cardiometabolic biomarkers (low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], total cholesterol, fasting glucose, triglycerides, triglyceride-glucose [TyG] index).
RESULTS: Of the 755 participants, the mean age was 68.5 years, and 68.2% were women. The proportion of participants with any IC impairment increased with age: 63.2% for those aged 50-64, 65.8% for those aged 65-74, and 74.7% for those aged ≥75 years based on ICOPE Step 1. For ICOPE Step 2, the proportions were 59.9%, 56.9%, and 64.0%, respectively. Impairments in locomotion and cognition were significantly higher in the oldest age group (≥75 years). Adjusted for covariates, IC impairment (ICOPE Step 2) was associated with higher levels of neutrophil count (β = 3.17, p = 0.015) and NLR (β = 0.34, p = 0.021) in those aged 50-64 years, and higher levels of monocyte count in those aged 65-74 years (β = 0.65, p = 0.001) and ≥75 years (β = 0.68, p = 0.037).
CONCLUSIONS AND RELEVANCE: In conclusion, IC impairments were associated with alterations in specific inflammatory biomarkers, suggesting potential interactions between IC, age, and inflammatory processes. Longitudinal studies are warranted to establish causal relationships and elucidate the underlying mechanisms linking IC impairments, immune dysregulation, and the aging process.
Key words: Intrinsic capacity, Integrated Care for Older People (ICOPE), cardiometabolic risk, immune resilience, healthy longevity.
Introduction
Population aging, a global phenomenon marked by a shift in demographics towards older populations, presents a multifaceted challenge for developed and developing countries alike, and Taiwan is no exception (1). In response to the multidimensional challenges posed by population aging, the World Health Organization (WHO) published the World Report on Aging and Health, advocating for healthy aging as a new paradigm to support healthcare systems and societies. Subsequently, the WHO introduced the Integrated Care for Older People (ICOPE) framework, which operationalizes the measurement of intrinsic capacity (IC) and systematically promotes healthy aging (2). At its core, the ICOPE strategy seeks to uphold optimal functional abilities among older adults while mitigating or compressing care dependency as much as possible. Central to this approach is the concept of IC, which encompasses an individual’s physical and mental capabilities crucial for promoting healthy aging (3).
To effectively identify individuals at risk of care dependency, the ICOPE healthcare pathway begins with ICOPE Step 1 (4, 5), which involves screening for failing tasks across six domains of IC: locomotion, vitality, visuality, hearing loss, cognitive function, and psychological well-being. It is important to note that these identified failing tasks do not constitute clinical diagnoses; rather, they serve as indicators that may stem from underlying health conditions. Following the screening process, the results act as triggers for subsequent steps within the ICOPE pathway. This includes conducting more in-depth assessments (Step 2) to pinpoint the root causes of IC decline and establishing personalized care plans tailored to the individual’s specific needs and circumstances. Existing studies have reported that each of these elements, alone or in combination, significantly predicts subsequent disability, dementia, and other adverse health outcomes (4, 6-9).
Among all health outcomes and pathophysiological characteristics investigated, the link between IC and biomarkers has attracted attention but remained limited and inconclusive (10, 11). During the aging process, the cardiometabolic risk, as assessed by serum lipid and glycemic profiles, exhibits significant alterations that profoundly impact on healthy longevity. With advancing age, alterations in lipid metabolism often present with an increase in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides, alongside a decline in high-density lipoprotein cholesterol (HDL-C) levels. These changes accelerate the development of atherosclerosis and cardiovascular disease, which are major contributors to morbidity and mortality in older adults (12). Similarly, glycemic regulation becomes less efficient with age, resulting in a higher prevalence of impaired glucose tolerance and type 2 diabetes among older individuals (13, 14). These metabolic changes are closely intertwined with age-related conditions, and the triglyceride-glucose index has garnered extensive research interest due to its associations with metabolic health and insulin resistance (14).
Complementing the well-known metabolic alterations, age-associated dysregulation of the immune system, including immunosenescence and inflammaging, has emerged as a critical area of research. Immunosenescence, marked by a dampened responsiveness to antigens and/or cellular immunity, and inflammaging, characterized by a chronic propensity for low-grade inflammation, represent distinct but interconnected facets of age-related immune dysfunction (10, 11, 15-17). Recognizing the heterogeneity of aging, the concept of immune resilience has recently emerged, highlighting individual potentials in immune reserve, particularly the ability to resist environmental challenges (18). Specifically, the neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) are identified as biomarkers of changes in the innate and adaptive immunity during aging(19, 20).
Despite established links between IC and inflammatory biomarkers, elucidating the interplay between IC, immunological dysfunction, and metabolic alterations during aging remains crucial for promoting overall health resilience and facilitating a more active and fulfilling late-life experience in older adults. Therefore, the aim of this study is to investigate the associations between IC (both ICOPE Step 1 and Step 2) and aging-related biomarkers (including inflammatory, cardiometabolic biomarkers and immune resilience) in community-dwelling middle-aged and older adults.
Methods
Study subjects
Data extracted from 755 participants enrolled in The Gan-Dau Healthy Longevity Plan (21-24), a community-based survey overseen by the Taipei Municipal Gan-Dau Hospital and situated within the Gan-Dau community of the Beitou District in Taipei City, were accessed for analytical purposes. The Gan-Dau locality is home to an estimated population of 55,000 individuals, characterized notably by a demographic composition where individuals aged 65 years and older constitute more than 18.5% of the population as of 2022. The primary objective of The Gan-Dau Healthy Longevity Plan is to implement the ICOPE strategies with well-evidenced interventions and enhance IC and well-being of the community, in accordance with the principles delineated in the “UN Decade of Healthy Ageing”. Employing tailored methodologies, the plan is designed to realize its goals effectively (25).” Based on the “ICOPE: guidance for person-centred assessment and pathways in primary care” released by the WHO (26), which adopted a life-course approach, the IC assessment is applied mainly but not limited to older adults. To better capture the life-course risk of IC impairments, the Gan-Dau Healthy Longevity Plan included community-dwelling people aged 50 years and older to promote healthy aging (21-24).
The cohort in this study was identified from the 2022 Gan-Dau Healthy Longevity Plan survey, in which 810 participants were enrolled. Data in the survey were collected via face-to-face interviews that included demographics, household information, chronic medical conditions, and assessments of physical, functional, and mental health. Among them, we excluded participants with incomplete data in demographics (n=16) and each domain of IC measurements (n=39). As we planned to examine how IC impairments and their associations varied during the aging process, study participants were further stratified by age (50–64, 65-74, 75+ years old).
Ethics statement
The design and implementation of this study adhered to the ethical principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was duly sanctioned by the institutional review board of National Yang Ming Chiao Tung University (No. YM110149F), and the participants provided their written informed consent prior to their enrolment in the study.
Domains of Intrinsic capacity: ICOPE Step 1
We operationalized an IC screening tool similar to the ICOPE Step 1 (26), based on the detection of IC impairments (fail tasks). Specifically, we adhered closely to the definition of the ICOPE Step 1 tool for three domains:
Locomotion: To assess mobility, participants were instructed to perform a 6-meter timed walk, aiming for a speed of less than 1.0 meters per second. This definition is also accordant to the Asian Working Group for Sarcopenia (AWGS) 2019 consensus (27). Since the Gan-Dau Healthy Longevity Plan survey cohort included participants under 65 years old, the cutoff for defining task failure in the locomotion domain for participants aged 50-64 years was defined as the lowest 20 percentiles of the group’s 6-meter timed walk performance. For men, the cutoff for people aged 50-64 years was set at less than 1.08 meters per second, and for women, it was less than 1.04 meters per second.
Vitality: Self-reported indicators of weight loss or appetite loss were utilized to gauge vitality and nutritional status.
Cognition: Time and space orientation plus word recall (28).
Due to data availability, we had to adapt the operationalization of vision, auditory, and psychological domains.
Vision: For participants answering “yes” to any of the question: 1) “(Even if wearing glasses), do you have visual problems in seeing far or reading, 2) or eye diseases?”;
Hearing: For participants answering “yes” to the question “When talking to someone, the other person often needs to speak louder or speak again?”
Psychological well-being: We used the Geriatric Depression Scale (GDS-5), a widely recognized and validated instrument for detecting depression in older adults (29). For participants answering “yes” to the item 2 of GDS-5, “Do you feel bored most of the time?”, or responding “yes” to the item 5 of the GDS-5 “Do you feel hopeless most of the time?”. Three experts (two geriatricians, one general practitioner, and one researcher in clinical gerontology) judged these GDS items as the closest ones to the ICOPE screening.
Domains of Intrinsic capacity: ICOPE Step 2
For ICOPE Step 2, the following definitions were adopted. The definitions of locomotion, vision and auditory were the same as ICOPE Step 1 in this study, but eventually, the Step 2 assessment were used based on clinical scenarios.
Vitality: MNA-SF score: 0–16, with higher scores indicative of better nutrition. The MNA-SF < 12 was selected as a cut-off (30).
Cognition: Mini-mental status examination (MMSE) (31). The cut-off point of the MMSE for people with any level of education is 24, and for those who have never attended school is 14 (32).
Psychological well-being: Geriatric Depression Scale (GDS-5). Participants were asked to answer “yes” (scored as “1”) or “no” (scored as “0”) for each item. Their responses were summed to create an overall depression score ranging from 0 to 5, with a higher score indicating a higher level of depression. The GDS-5 >= 2 was selected as a cut-off (29).
Age-related biomarkers
After a 10-hour overnight fast, venous blood samples were obtained from all participants to assess age-related biomarkers, including immune biomarkers (albumin, white blood cell (WBC) count, neutrophil, lymphocyte, platelet, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR)and platelet-to-lymphocyte ratio (PLR)) and cardiometabolic biomarkers LDL-C, HDL-C, total cholesterol, fasting glucose, triglycerides, and triglyceride-glucose (TyG) index (TyG index = ln [Fasting triglyceride (mg/dl) × fasting glucose (mg/dl)]/2).
Demographic characteristics
The following demographic characteristics for each study participant were collected: sex (male, female), age (categorized as 50-64, 65-74, and 75+ years), educational years (<6, 7-9, >10), marital status (married and others), living status (alone, with spouse or other family members), religion belief (yes/no), employment status (yes/no), lifestyle behaviors (current smoker and current drinking), and number of self-reported chronic medical conditions (0, 1, 2+).
Statistical analysis
All analyses in this study were stratified by age. Comparisons of demographics between those with and without IC impairments (ICOPE Step 1 and Step 2) were performed with the chi-square or Fisher’s tests for categorical variables and t-tests for continuous variables when appropriate. Multivariate generalized linear model (GLM) was further used to examine the association between IC impairments (ICOPE Step 1 and Step 2) and changes in biomarkers, with the adjustment of sex, educational years, marital status, living status, religion belief, employment status, lifestyle behaviors (current smoker and current drinking), and number of self-reported chronic medical conditions (0, 1, 2+). For all tests, a two-tailed p value <0.05 was considered statistically significant. All data were analyzed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study cohort and demographics
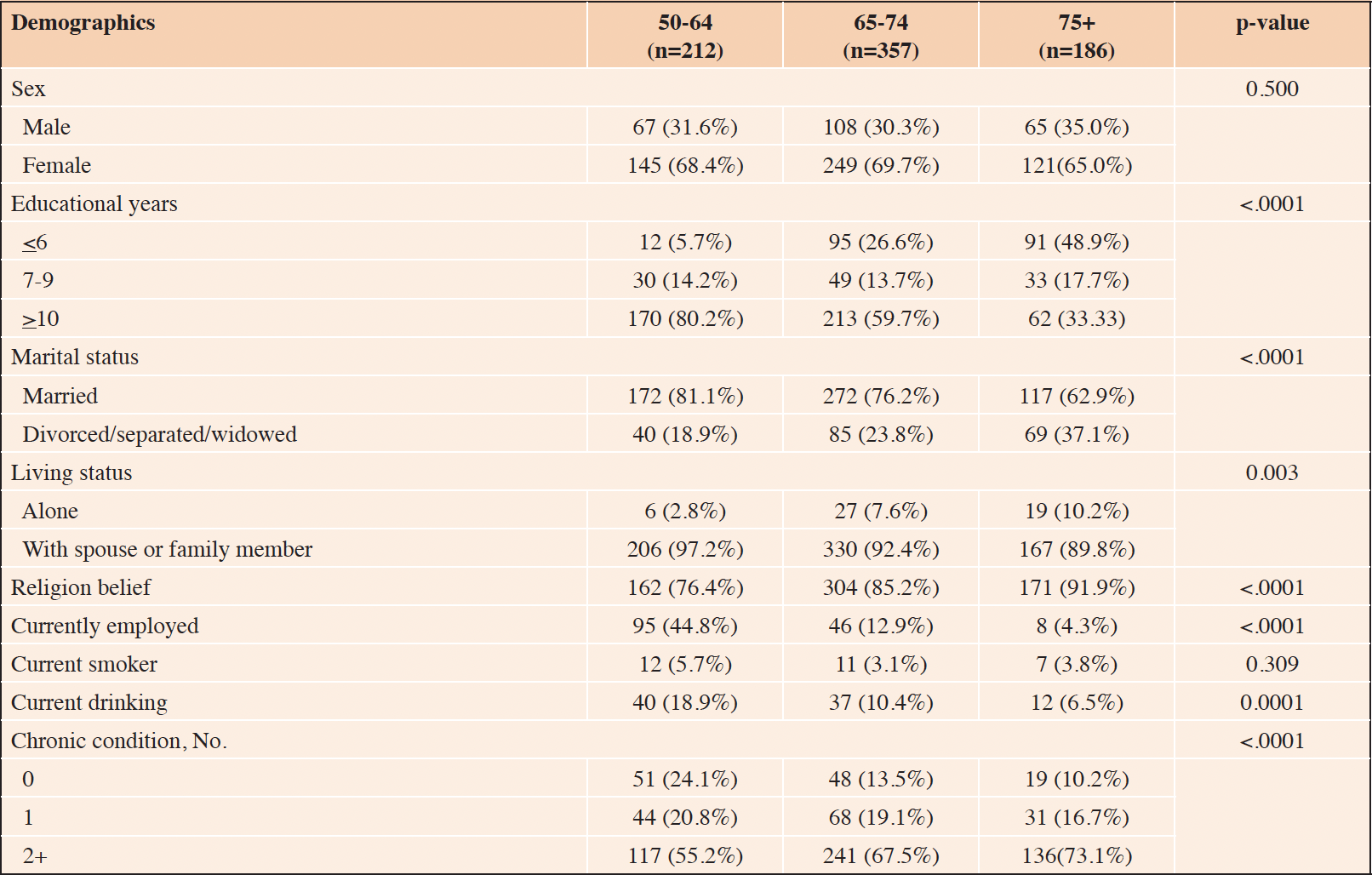
Table 1 summarizes the demographic characteristics of the study participants stratified by three age groups (aged 50-64, 65-74 and 75+). In total, 755 participants (aged 50-64 (n=212), 65-74 (n=357) and, 75+ (n=186)) were included in the study. The sex distribution was comparable within each age strata, with female as the dominant gender (aged 50-64 (68.4%), 65-74 (69.7%) and, 75+ (65.0%), p=0.500).
The proportion of married participants decreased with age (aged 50-64 (81.1%), 65-74 (76.2%) and, 75+ (62.9%), p<0.001) and percentage of people living alone increased with age (aged 50-64 (2.8%), 65-74 (7.6%) and, 75+(10.2%), p=0.003). Study participants in the highest age strata had the highest proportion of having religion belief (91.9%, p<0.001). The employment rate decreased by age, with only 4.3% of those aged 75+ being currently employed (p<0.001). Those aged 75+ were less likely to be current drinkers (aged 50-64 (18.9%), 65-74 (10.4%) and, 75+ (6.5%), p<0.001).
Intrinsic capacity (IC) impairments by ICOPE Step 1 and Step 2
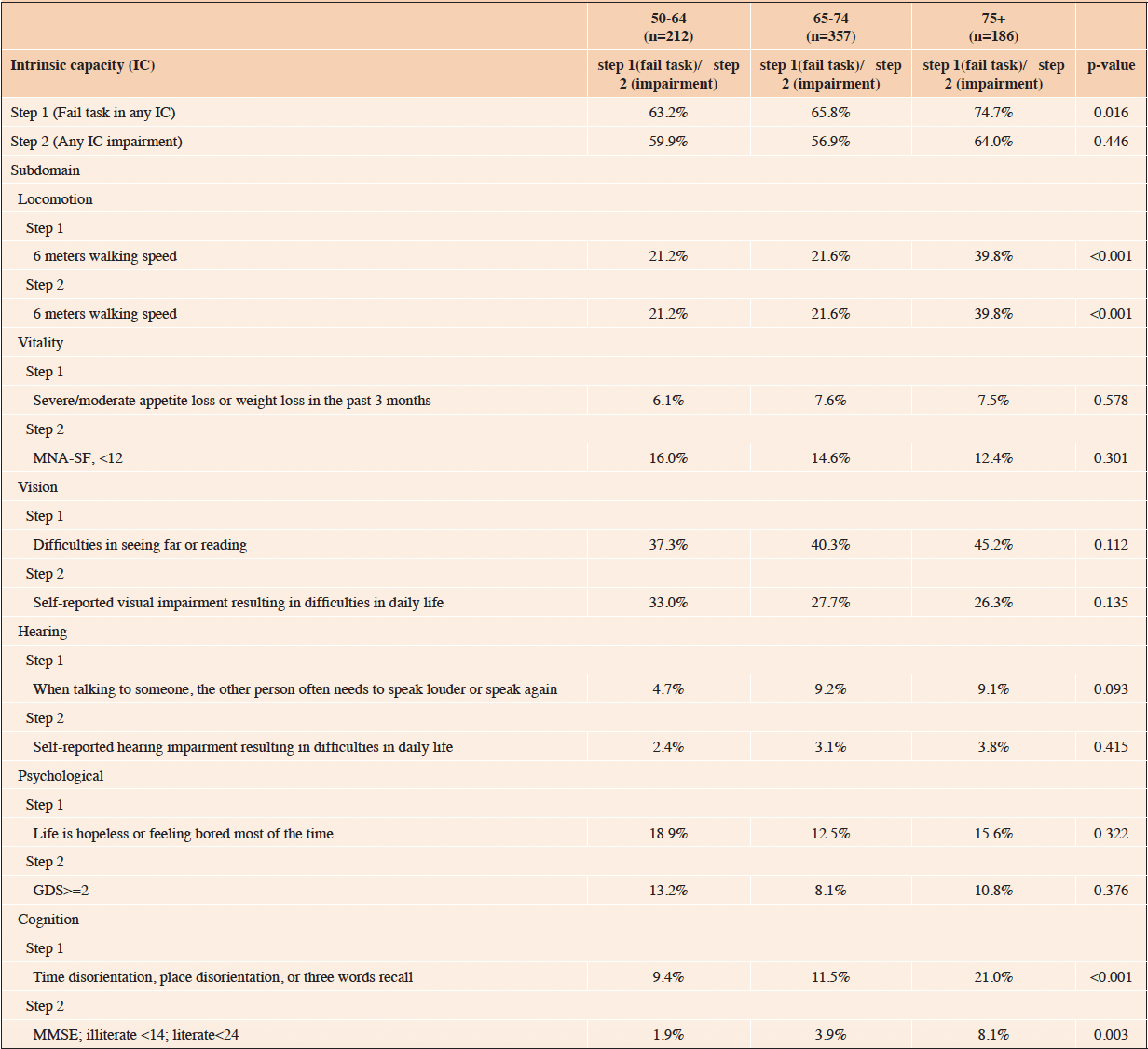
Table 2 revealed the IC impairments across different age groups. Overall, the proportion of IC impairments increased with age. For ICOPE Step 1 screening, the proportion of any IC impairment was 63.2%, 65.8%, and 74.7% for those aged 50-64, 65-74, and 75+, respectively. Those with any IC impairment identified by ICOPE Step 2 were 59.9%, 56.9%, and 64.0% for those aged 50-64, 65-74, and 75+, respectively.
For locomotion impairment (measured by the 6-meter walking speed in both Step 1 and Step 2), the highest proportion was found in those aged 75+ (39.8%) versus approximately 20% in those aged 50-64 and 65-74. For most of the IC domains, the proportion of impairments (screened based on ICOPE Step 1) increased with age. These include vision (aged 50-64 (37.3%), 65-74 (40.3%) and, 75+ (45.2%)), and cognition (aged 50-64 (9.4%), 65-74 (11.5%) and, 75+ (21.0%)), although only cognition domain reached statistical significance (p<0.001). Similar findings were found in the cognition domain identified by the ICOPE Step 2 (MMSE, aged 50-64 (1.9%), 65-74 (3.9%) and, 75+ (8.1%), p=0.003).
Despite not reaching statistical significance, we noticed that those aged 50-64 had the highest impairment in psychological well-being identified by ICOPE Step 1 (18.9%) and Step 2 (13.2%). Noteworthy, those aged 50-64 also had the highest impairment in vitality (16.0%) identified by the ICOPE Step 2 (MNA-SF), which deserves further investigation.
IC= intrinsic capacity, MNA-SF= Mini Nutritional Assessment-short form, GDS= Geriatric Depression Scale, MMSE= Mini-mental status examination
The association between IC and biomarkers
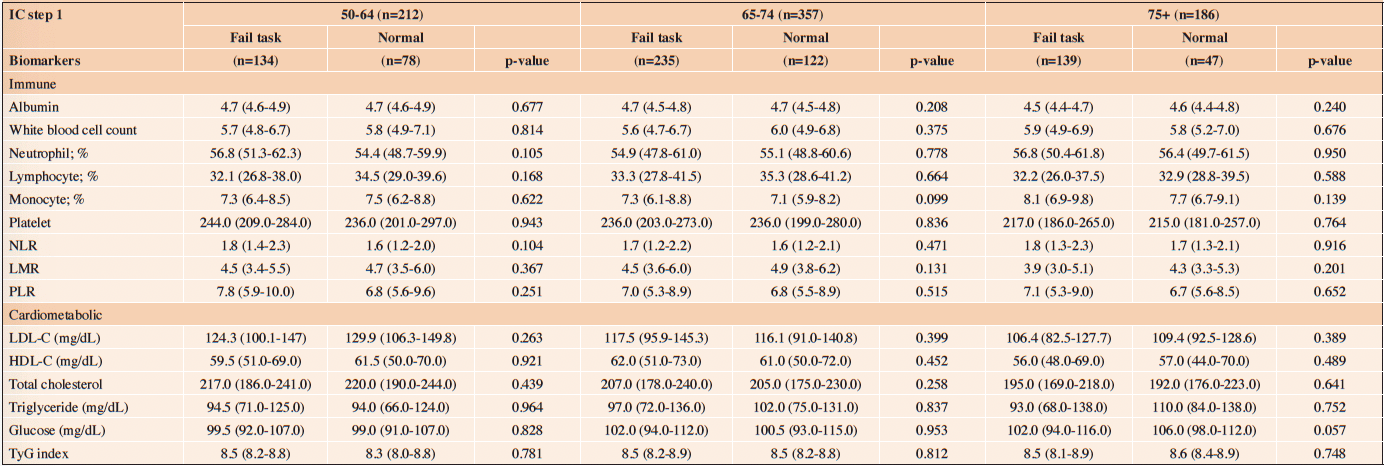
Table 3 (a) reveals the association between IC (ICOPE Step 1) and biomarkers. The levels of biomarkers were all comparable between those who failed task (ICOPE Step 1) and those who did not. Unadjusted and multivariate GLM also yield no statistically significant associations between IC (ICOPE Step 1) and biomarkers (Table 4 (a)).
NLR=Neutrophil-to-lymphocyte ratio; LMR=Lymphocyte-to-monocyte ratio; PLR=Platelet-to-lymphocyte; TyG =Triglyceride-glucose
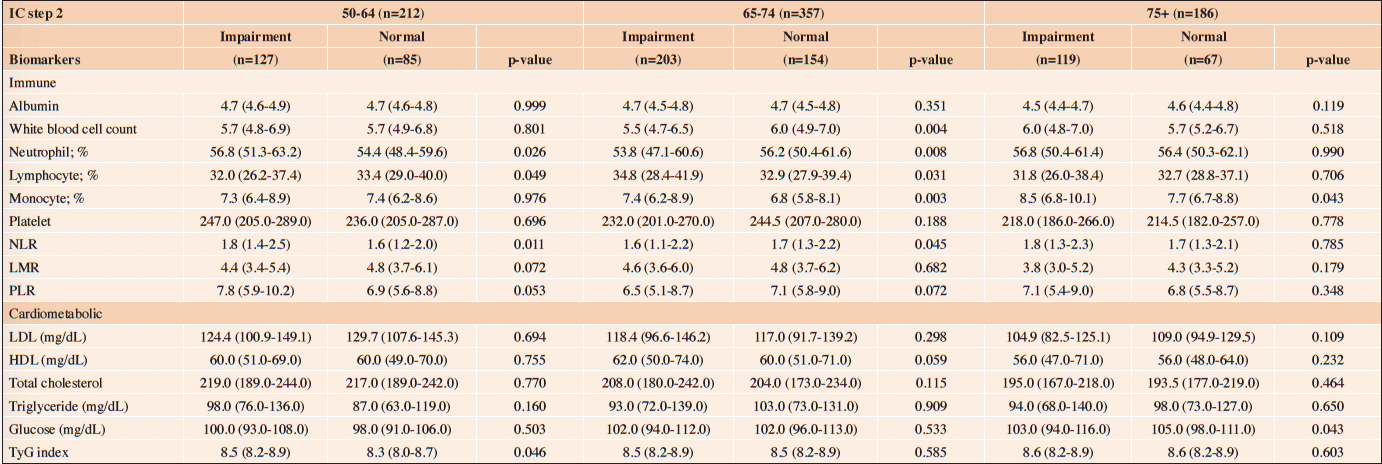
Table 3 (b) reveals the association between IC impairment (ICOPE Step 2) and biomarkers. Among those aged 50-64, a higher level of neutrophil count (56.8% vs. 54.4%, p=0.026) and NLR (1.8 vs. 1.6, p=0.011) were found in those with IC impairments (ICOPE step 2). Those with IC impairments (ICOPE Step 2) also had higher level of TyG index (median 8.5 (IQR 8.2-8.9) vs. 8.3 (8.0-8.7), p=0.046). Multivariate GLM indicated that IC impairment (ICOPE Step 2) was associated with higher level of neutrophil count (β: 3.17, p=0.015) and NLR (β: 0.34, p=0.021). (Table 4 (b))
NLR=Neutrophil-to-lymphocyte ratio; LMR=Lymphocyte-to-monocyte ratio; PLR=Platelet-to-lymphocyte; TyG =Triglyceride-glucose
Among those aged 65-74, a higher level of lymphocyte count (34.8% vs. 32.9%, p=0.031) and monocyte count (7.4% vs. 6.8%, p=0.003) were found in those with IC impairments (ICOPE Step 2) (Table 3 (b)). Multivariate GLM indicated that IC impairment (ICOPE Step 2) was associated with higher level of monocyte count (β: 0.65, p=0.001). (Table 4 (b)) Among those aged 75+, multivariate GLM also indicated that IC impairment (ICOPE Step 2) was associated with higher levels of monocyte count (β: 0.68, p=0.037).
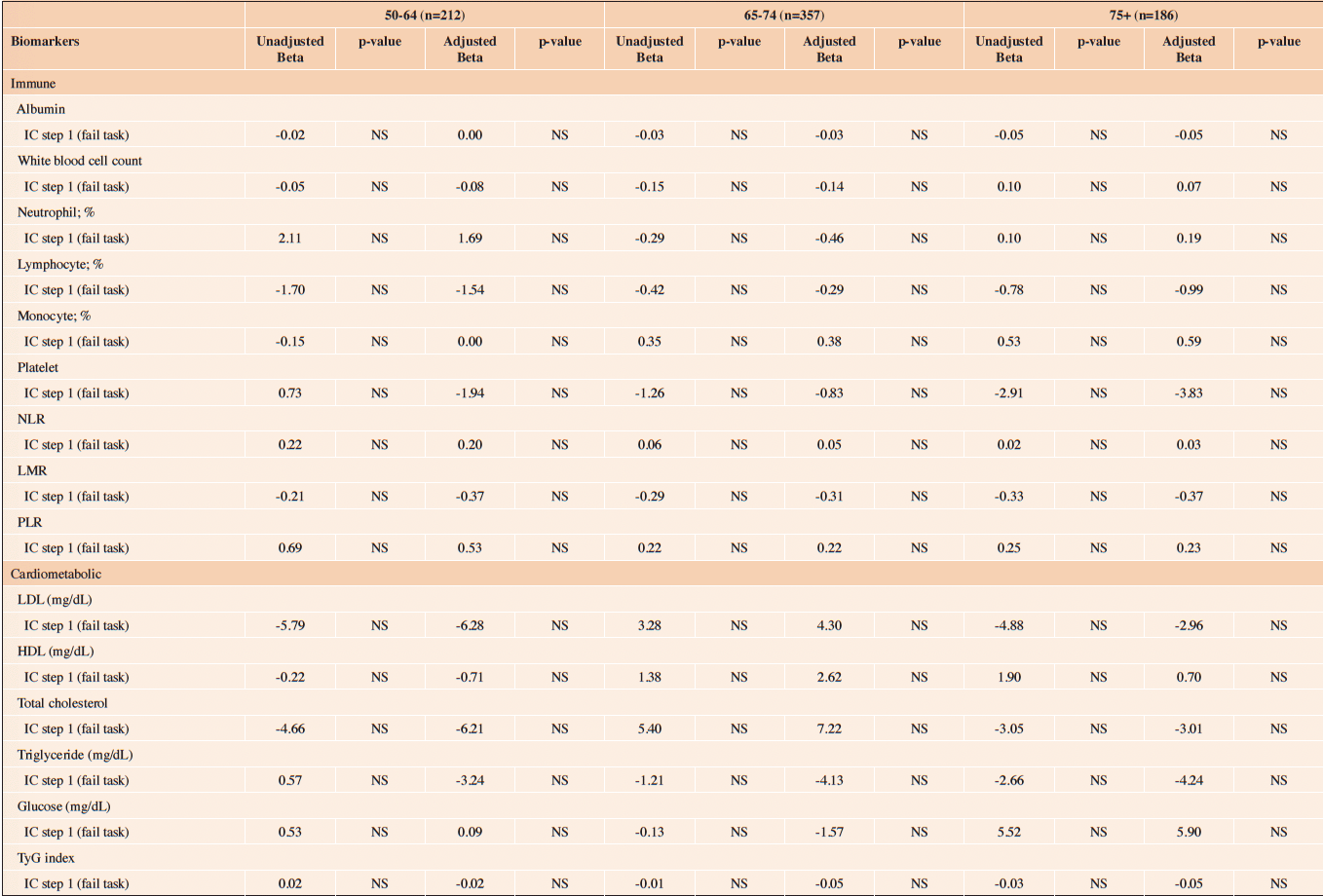
Table 4 (a). The association between IC impairment (ICOPE step 1) and level of biomarkers; stratified by age
NLR=Neutrophil-to-lymphocyte ratio; LMR=Lymphocyte-to-monocyte ratio; PLR=Platelet-to-lymphocyte; TyG =Triglyceride-glucose; NS=Not statistically significant
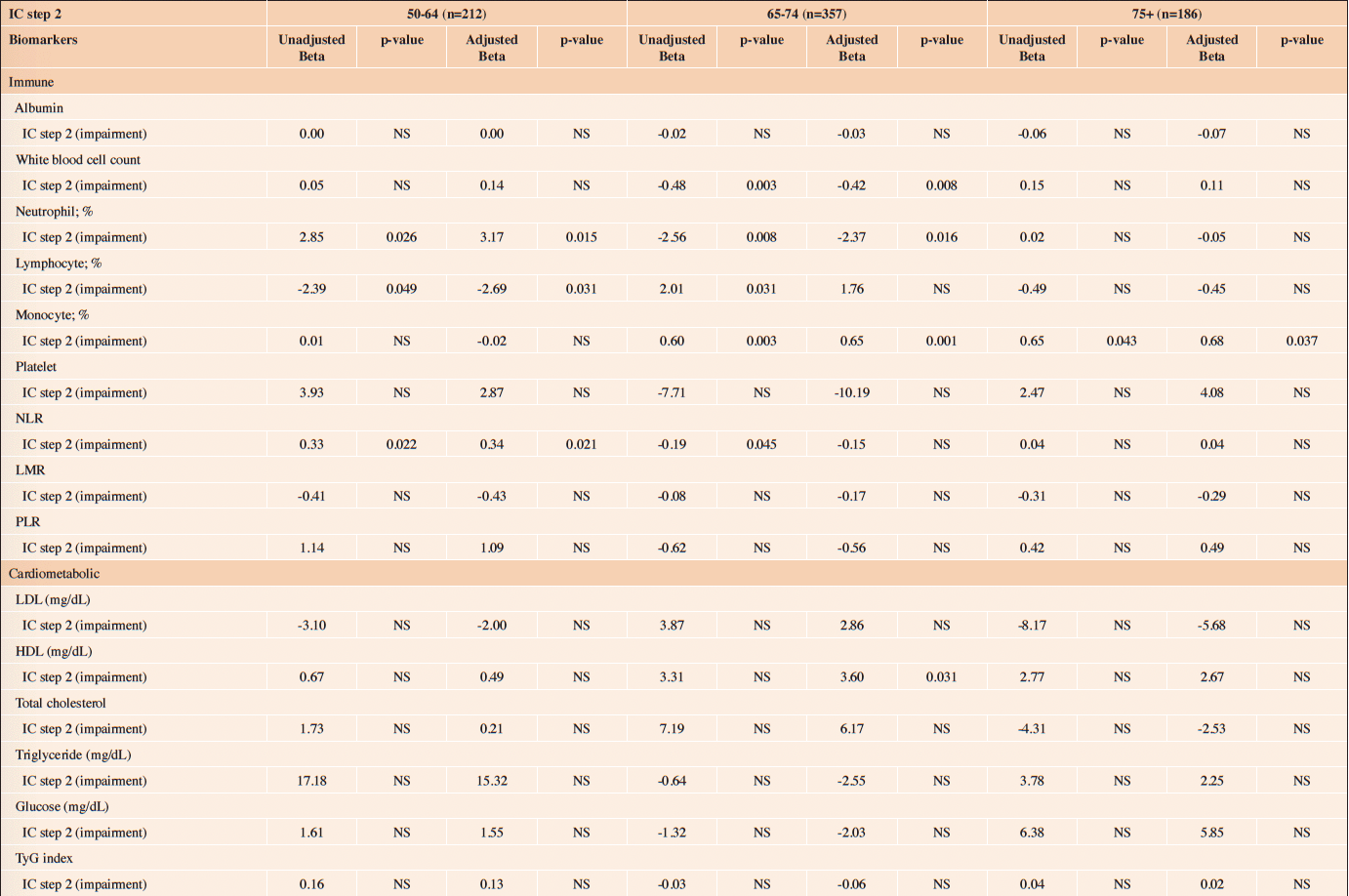
Table 4 (b). The association between IC impairment (ICOPE step 2) and level of biomarkers; stratified by age
NLR=Neutrophil-to-lymphocyte ratio; LMR=Lymphocyte-to-monocyte ratio; PLR=Platelet-to-lymphocyte; TyG =Triglyceride-glucose; NS=Not statistically significant
Discussion
This study found an age-dependent trend of IC impairments in both ICOPE Step 1 and 2. For example, over 74% of those aged 75+ had limitations in at least one domain of IC, compared to 63% of those aged 50-64. Examining specific areas, the prevalence of impairments in locomotion and cognition were significantly higher in the oldest age group. We also looked at associations between IC and various biomarkers. While no significant connection was found for people in all age groups combined, further analysis revealed some interesting trends. Among the oldest participants (aged 75+), those with IC impairments had higher levels of monocyte count. Similarly, for people between 65-74, IC impairment was linked to elevated monocyte count. In the youngest group (aged 50-64), individuals with IC limitations showed increased levels of neutrophil count and NLR. A recent study reported that higher NLR and MLR were associated with transitions from healthy to pre-frail/frail states and increased mortality risk, independent of other factors (20). The observed association between NLR/MLR and health outcomes may reflect impaired immune resilience rather than chronic low-grade inflammation. This interpretation aligns with the observed IC impairments in participants with high NLR/MLR.
Our study offers a comprehensive IC screening (ICOPE Step 1) and assessment (ICOPE Step 2) in a community-dwelling middle-aged and older adult, which is seldom reported in existing literatures. The ICOPE framework is envisioned to function in a multiple-tiered approach. Step 1 offers self-assessment tools, empowering older adults to gain insights into their IC. Conversely, Step 2 is geared towards clinical settings. Here, healthcare professionals can employ more specific assessments tailored to individual needs, enabling a more comprehensive evaluation of IC to inform subsequent care plans. To the best of our knowledge, very few programs are promoting ICOPE in the real-world community instead of findings from existing research cohort and the Gan-Dau Healthy Longevity Plan (Taiwan) is one of them (21). In addition, only few studies with limited, non-generalizable samples have compared ICOPE Step 1 and 2 assessment results (33). Prior investigations explored the relationship between IC impairment and associated biomarkers are also very limited.
A study in Singapore used a relatively small sample comparing ICOPE Steps 1 and 2 assessments for IC in older adults. Both steps found a high prevalence of IC impairments (over 66%), but Step 2 offered a more detailed picture, in which locomotion and cognition were the most commonly affected domains (around 40% and 25%, respectively) compared to the general screening of Step 1 (34). Rodríguez-Laso, et al evaluated the clinical efficacy of ICOPE screening tool and found that individual IC domain (e.g. locomotion domain), but not the overall IC, predicted dependence and hospitalization over 3 years. In particular, the cognition domain showed weaknesses in predicting adverse outcome (35). However, another study investigated whether ICOPE Step 1 cognition assessment could predict dementia in older adults, and found that forgetting the weekday (time disorientation) tripled the risk of dementia within 5 years (hazard ratio (HR) 3.11, 95% CI: 1.18-8.17), while mistakes recalling words also significantly increased the risk of dementia (HR for two mistakes = 3.50, 95% CI: 1.49-8.26; HR for three mistakes = 4.28, 95% CI: 1.60-11.46) (36). Our findings mirrored previous observations that ICOPE Step 1 detected a higher prevalence of overall IC impairment, while Step 2 identified individuals with potentially specific disease risks requiring further workup. Locomotion and cognition were the most commonly affected domains, exhibiting an age-related decline. While prior ICOPE research has primarily focused on older adults, our findings in participants aged 65 and above align with existing literature. However, the middle-aged group showed a higher prevalence of vitality and psychological well-being impairments compared to older groups, suggesting potential factors like unidentified psychological stress, depressive moods, or intake issues specific to this age range. In this age group of our study, females significantly outnumber males and they did show stronger worries about life, less resilient and less happiness (24, 37), which might be an explanation regarding the phenomenon we revealed. However, it needs further study to provide proper answer.
A study using the data of UK Biobank indicated that lower IC significantly predicted higher risk of cardiovascular disease (CVD) and even death from CVD over 10 years (38). Moreover, in a study of over 1,200 older adults, researchers found that higher levels of inflammatory markers in the blood (CRP, IL-6, TNFR-1, MCP-1, GDF-15) were associated with both lower baseline IC and a faster decline in IC over 4 years (39). The same research group further demonstrate that while IC exhibits heterogeneous longitudinal trajectories, higher concentrations of inflammatory markers (IL-6, TNFR-1) and the mitochondrial dysfunction marker GDF-15 are associated with an increased risk of accelerated decline in overall physical and mental health among older adults (11). These findings suggest a potential link between cardiometabolic risk, chronic inflammation and reduced physical and mental capacity in the aging process. Our previous study found that people with lower IC had higher levels of inflammatory markers (IL-6), lower levels of albumin and folate, and were more likely to carry the APOE ε4 gene (especially those 60 and older) (9).
In the aging process, some individuals exhibit a remarkable ability to maintain optimal immune resilience throughout their lives, even in the face of aging and inflammatory stressors. They preserve and rapidly restore immune functions that promote disease resistance and control inflammation. The balance between CD8+ and CD4+ T-cell levels, as well as gene expression signatures tracking immunocompetence versus inflammation, may represent indicators of immune resilience. In other words, maintaining the optimal ratio of these T-cell types and monitoring gene expression patterns related to immune function and inflammation could be crucial markers of a robust and adaptable immune system (18). In addition to the aforementioned items, the NLR and MLR have been reported as markers of immune resilience that higher NLR and MLR significantly predicted frailty and death (18). In this study, NLR was higher in individuals with IC impairment (Step 2) compared to those without in the 50-64 and 65-74 age groups, but not in the 75+ age group. However, there was no consistent association between IC impairment and the levels of immune biomarkers across age groups in the regression model. These findings suggest that the relationship between immune resilience and IC impairment may not be straightforward and may vary with age. The association between immune resilience and IC impairments over time may be influenced by additional, yet unidentified, confounding factors. Nonetheless, these findings suggest that aging and IC impairments are not solely attributable to chronic inflammation. Rather, a complex interplay between these factors and immune resilience appears to underlie the observed associations.
While this study offers valuable insights, inherent limitations associated with the study design and data limited the ability to definitively establish causal links between IC and outcomes of interest. First, the cross-sectional nature of the study precludes the determination of causality between IC impairments and age-related biomarkers. Follow-up studies are warranted to further validate our findings. Second, the age strata employed in the cross-sectional design might not adequately capture the longitudinal effects, as middle-aged individuals may be undergoing specific life-course environmental stressors not accounted for. However, one recent study building IC throughout adulthood (aged 20-102) and their association with clinical outcomes (5) has found higher IC levels in young and middle age and markedly lower levels after the age of 65 years. In this study, all assessments of IC (including self-reported ones) were operationalized exactly the same for those aged 20-49, 50-69, 70+, which support the operational definitions of our study. Nevertheless, future studies may be warranted to investigate the validity of these assessments in different age groups. Third, while the absence of T-cell subset data precluded a comprehensive assessment of standardized immune resilience, NLR and MLR have been established as effective and practical markers in community-based studies. Acknowledging the methodological limitations, this study presents a novel investigation into the association between IC impairment and immune resilience. This finding paves the way for further research exploring the potential for balancing the detrimental effects of immune senescence and inflammaging during the aging process.
Conclusion
In conclusion, this study revealed a significant association between IC impairments and alterations in specific inflammatory biomarkers, but not cardiometabolic risk profiles. This finding suggests a potential interplay between IC, chronic inflammation and immune resilience. To establish causal relationships and illuminate the intricate mechanisms linking IC impairments, immune dysregulation, and the aging process, future longitudinal studies and intervention studies are warranted.
Funding Sources: This research was funded by the Taiwan Ministry of Science and Technology (MOST 110-2634-F-010-001), the Taiwan National Science and Technology Council (NSTC 111-2622-8-A49-019-IE, NSTC 112-2923-B-A49-002-MY2), the National Health Research Institutes (NHRI 13A1-CG-CO-05-2426-3), and the Interdisciplinary Research Center for Healthy Longevity of National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, and Taipei Municipal Gan-Dau Hospital. The funding source had no role in conducting this study, including study design, data collection and analysis, manuscript preparation and review, and the decision to submit the manuscript for publication.
Conflict of Interest: The authors declare no conflicts of interest.
Author contribution: Chen ZJ, Chen LK, and Hsiao FY designed the research. Chen ZJ, Chen LK, and Hsiao FY drafted and prepared the manuscript. Chao WF and Hsiao FY analyzed the data. Chen ZJ, Meng LC, Lu WH, Tung HH, Chen LK, and Hsiao FY provided critical methodological input. Meng LC, Chen LK, and Hsiao FY provided critical statistical input. Chen ZJ, Tung HH, Chen LK, and Hsiao FY contributed to the clinical interpretation. All authors drafted the article, revised it critically for important intellectual content, and approved the final version for publication.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Chen LK. Advancing the journey: Taiwan’s ongoing efforts in reshaping the future for aging populations. Arch Gerontol Geriatr. 2023;113:105128.
2. Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145-54.
3. Beard JR, Si Y, Liu Z, Chenoweth L, Hanewald K. Intrinsic Capacity: Validation of a New WHO Concept for Healthy Aging in a Longitudinal Chinese Study. J Gerontol A Biol Sci Med Sci. 2022;77(1):94-100.
4. Lee WJ, Peng LN, Lin MH, Loh CH, Hsiao FY, Chen LK. Intrinsic capacity differs from functional ability in predicting 10-year mortality and biological features in healthy aging: results from the I-Lan longitudinal aging study. Aging (Albany NY). 2023;15(3):748-64.
5. Lu WH, Rolland Y, Guyonnet S, de Souto Barreto P, Vellas B. Reference centiles for intrinsic capacity throughout adulthood and their association with clinical outcomes: a cross-sectional analysis from the INSPIRE-T cohort. Nat Aging. 2023;3(12):1521-8.
6. Lee WJ, Peng LN, Lin MH, Loh CH, Hsiao FY, Chen LK. Intrinsic capacity and multimorbidity predicting incident disability-Insights from the I-Lan Longitudinal Aging Study. Arch Gerontol Geriatr. 2024;121:105357.
7. Meng LC, Chuang HM, Lu WH, Lee WJ, Liang CK, Loh CH, et al. Multi-Trajectories of Intrinsic Capacity Decline and Their Impact on Age-Related Outcomes: A 20-Year National Longitudinal Cohort Study. Aging Dis. 2023.
8. Meng LC, Hsiao FY, Huang ST, Lu WH, Peng LN, Chen LK. Intrinsic Capacity Impairment Patterns and their Associations with Unfavorable Medication Utilization: A Nationwide Population-Based Study of 37,993 Community-Dwelling Older Adults. J Nutr Health Aging. 2022;26(10):918-25.
9. Meng LC, Huang ST, Peng LN, Chen LK, Hsiao FY. Biological Features of the Outcome-Based Intrinsic Capacity Composite Scores From a Population-Based Cohort Study: Pas de Deux of Biological and Functional Aging. Front Med (Lausanne). 2022;9:851882.
10. Lee WJ, Peng LN, Lin MH, Kim S, Hsiao FY, Chen LK. Enhancing Intrinsic Capacity and Related Biomarkers in Community-Dwelling Multimorbid Older Adults Through Integrated Multidomain Interventions: Ancillary Findings From the Taiwan Integrated Geriatric (TIGER) Trial. J Am Med Dir Assoc. 2023.
11. Lu WH, Guyonnet S, Martinez LO, Lucas A, Parini A, Vellas B, et al. Association between aging-related biomarkers and longitudinal trajectories of intrinsic capacity in older adults. Geroscience. 2023;45(6):3409-18.
12. Hsu CY, Chen WJ, Lin HJ, Chen HM, Yang YH, Chen WJ, et al. Higher risk of future events, mortality and greater healthcare use among patients with increasingly recurrent atherosclerotic cardiovascular disease events in Taiwan: a retrospective cohort study. BMJ Open. 2023;13(7):e064219.
13. Huang ST, Chen LK, Hsiao FY. Clinical impacts of frailty on 123,172 people with diabetes mellitus considering the age of onset and drugs of choice: a nationwide population-based 10-year trajectory analysis. Age Ageing. 2023;52(7).
14. Chou MY, Huang ST, Liang CK, Peng LN, Lin YT, Hsiao FY, et al. All-cause mortality, cardiovascular mortality, major cardiovascular events and hypoglycaemia of patients with diabetes onset at an older age: results from the 10-year nationwide cohort study. Age Ageing. 2021;50(6):2094-104.
15. Liang CK, Lee WJ, Peng LN, Meng LC, Hsiao FY, Chen LK. COVID-19 Vaccines in Older Adults: Challenges in Vaccine Development and Policy Making. Clin Geriatr Med. 2022;38(3):605-20.
16. Lin HY, Hsiao FY, Huang ST, Chen YC, Lin SW, Chen LK. Longitudinal impact of distinct infection trajectories on all-cause mortality of older people in Taiwan: a retrospective, nationwide, population-based study. Lancet Healthy Longev. 2023;4(9):e508-e16.
17. Huang ST, Huang YC, Kuo E, Yang YM, Hsiao FY. Impacts of Catch-Up Immunization program with the 13-Valent pneumococcal Conjugate vaccine in Taiwan: Focus on age-stratified differences and high-risk population (2001-2015). Vaccine. 2022;40(43):6225-34.
18. Ahuja SK, Manoharan MS, Lee GC, McKinnon LR, Meunier JA, Steri M, et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat Commun. 2023;14(1):3286.
19. Bingol O, Ozdemir G, Kulakoglu B, Keskin OH, Korkmaz I, Kilic E. Admission neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio to predict 30-day and 1-year mortality in geriatric hip fractures. Injury. 2020;51(11):2663-7.
20. Pellegrino R, Paganelli R, Di Iorio A, Bandinelli S, Mussi C, Sparvieri E, et al. Lack of Immune Resilience Negatively Affects Physical Resilience: Results From the InCHIANTI Follow-Up Study. J Gerontol A Biol Sci Med Sci. 2024;79(5).
21. Chen LK. Gan-Dau Healthy Longevity Plan: The Community Model for Healthy Aging. Aging Med Healthc. 2022;13(3):98-101. doi:10.33879/amh.133.2022.09088.
22. Chen LK, Iijima K, Shimada H, Arai H. Community re-designs for healthy longevity: Japan and Taiwan examples. Arch Gerontol Geriatr. 2022:104875.
23. Lin SY, Lee WC, Kuo HY, Hsieh HJ, Lin YC, Chen PR, et al. Efficacy of a dementia-friendly integrated care network in mitigating cognitive decline and alleviating family care burden: Insights from the GAN-DAU healthy longevity plan. Aging Medicine and Healthcare, 14(3),162-168. http://doi.org/10.33879/amh.143.2023.07072. 2023.
24. Chen ZJ, Tung HH, Wang SY, Chou SS, Hsiao FY. Happiness and its determinants among community-dwelling middle-aged and older adults: Age- and sex-specific analysis in the Gan-Dau Healthy Longevity Plan. Aging Medicine and Healthcare, 14(4), 214-221. http://doi.org/10.33879/amh.144.2023.08080. 2023.
25. Amuthavalli Thiyagarajan J, Mikton C, Harwood RH, Gichu M, Gaigbe-Togbe V, Jhamba T, et al. The UN Decade of healthy ageing: strengthening measurement for monitoring health and wellbeing of older people. Age Ageing. 2022;51(7).
26. World Health Organization. Integrated care for older people (ICOPE): guidance for person-centred assessment and pathways in primary care. World Health Organization; 2019.
27. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300-7.e2.
28. Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S54-64.
29. Pocklington C, Gilbody S, Manea L, McMillan D. The diagnostic accuracy of brief versions of the Geriatric Depression Scale: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2016;31(8):837-57.
30. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-8.
31. Folstein MF, Folstein SE, McHugh PR. «Mini-mental state». A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-98.
32. Guo N-W, Liu H-C, Wong P-F, Liao K-K, Yan S-H, Lin K-P, et al. Chinese version and norms of the Mini-Mental State Examination. Rehabilitation Practice and Science. 1988;16(1):52-9.
33. Hsu PS, Lee WJ, Peng LN, Lu WH, Meng LC, Hsiao FY, et al. Safeguarding vitality and cognition: The role of sarcopenia in intrinsic capacity decline among octogenarians from multiple cohorts. J Nutr Health Aging. 2024;28(6):100268.
34. Leung AYM, Su JJ, Lee ESH, Fung JTS, Molassiotis A. Intrinsic capacity of older people in the community using WHO Integrated Care for Older People (ICOPE) framework: a cross-sectional study. BMC Geriatr. 2022;22(1):304.
35. Rodríguez-Laso Á, García-García FJ, Rodríguez-Mañas L. The icope Intrinsic Capacity Screening Tool: Measurement Structure and Predictive Validity of Dependence and Hospitalization. J Nutr Health Aging. 2023;27(10):808-16.
36. González-Bautista E, de Souto Barreto P, Andrieu S, Rolland Y, Vellas B. What day is today? Cognitive capacity and the risk of incident dementia in the context of integrated care for older people (ICOPE Step 1). Aging Clin Exp Res. 2021;33(11):3135-9.
37. Chen ZJ, Tang FP, Chang SY, Chung HL, Tsai WH, Chou SS, et al. Resilience-happiness nexus in community-dwelling middle-aged and older adults: Results from Gan-Dau Healthy Longevity Plan. Arch Gerontol Geriatr. 2024;116:105162.
38. Ramírez-Vélez R, Iriarte-Fernández M, Santafé G, Malanda A, Beard JR, Garcia-Hermoso A, et al. Association of intrinsic capacity with incidence and mortality of cardiovascular disease: Prospective study in UK Biobank. J Cachexia Sarcopenia Muscle. 2023;14(5):2054-63.
39. Lu WH, Gonzalez-Bautista E, Guyonnet S, Lucas A, Parini A, Walston JD, et al. Plasma inflammation-related biomarkers are associated with intrinsic capacity in community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2023;14(2):930-9.
The Author(s) 2024