L. Crombez1,2,3, A. Descamps1,4, H. Hirmz1,4, M. Lambert1,5, J. Calewaert1,2, D. Siluk6, M. Markuszewski6, M. Biesemans6, G. Petrella7, D. Cicero7, S. Cesaroni7, T. Stokowy8, G.K. Gerber9, C. Tataru9, P. Naumovski1,4, D. Elewaut1,3,10, C. Van De Looverbosch11, P. Calders11, N. Van Den Noortgate1,2, B. De Spiegeleer1,4, E. Wynendaele1,4, A. De Spiegeleer1,2,3
1. Translational Research in Immunosenescence, Gerontology and Geriatrics (TRIGG) group, Ghent University, Ghent, Belgium; 2. Department of Geriatrics, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium; 3. VIB Inflammation Research Center, Unit for Molecular Immunology and Inflammation, Ghent University, Ghent, Belgium; 4. Drug Quality and Registration (DruQuaR) group, Faculty of Pharmaceutical Sciences, Ghent University, Ghent, Belgium; 5. Department of Dental Health, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium; 6. Department of Biopharmacy and Pharmacodynamics, Faculty of Pharmacy, Medical University of Gdansk, Gdansk, Poland; 7. Department of Chemical Sciences and Technologies, University of Rome «Tor Vergata», Rome, Italy; 8. Scientific Computing Group, IT Division, University of Bergen, Bergen, Norway; 9. Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; 10. Department of Rheumatology, Faculty of Medicine and Health Sciences, Ghent University Hospital, Ghent, Belgium; 11. Department of Rehabilitation Sciences, Faculty of Medicine and Health Sciences, Ghent University Hospital, Ghent, Belgium.
Corresponding Author: Dr. A. De Spiegeleer: Department of Geriatrics, Faculty of Medicine and Health Sciences, Ghent University Hospital, C. Heymanslaan 10, 9000 Ghent, Belgium. Tel.: +32 (0) 9 332 84 67, Email: anton.despiegeleer@ugent.be
J Frailty Aging 2024;13(4)331-340
Published online October 10, 2024, http://dx.doi.org/10.14283/jfa.2024.75
Abstract
BACKGROUND: The gut microbiome is recognized as a pivotal factor in the pathophysiology of sarcopenia—a condition marked by the accelerated loss of muscle strength, mass and function with ageing. Despite this well-known gut-muscle axis, the potential links between other microbial ecosystems and sarcopenia remain largely unexplored. The oral microbiome has been linked to various age-related health conditions such as rheumatoid arthritis and colorectal cancer. However, its potential association with sarcopenia is unknown. The Saliva and Muscle (SaMu) study seeks to address this knowledge gap.
METHODS: The SaMu study comprises three sequential phases. In phase 1, a cross-sectional analysis will be conducted on a cohort of 200 individuals aged 70 years or older to examine the relationship between salivary microbiome and sarcopenia status. Participants will be recruited in the three main places of living: general community, assisted living facilities and nursing homes. The salivary microbiome composition will be evaluated utilizing shotgun metagenomics sequencing, while sarcopenia status will be determined through muscle mass (determined by whole-body bioelectrical impedance analysis and calf circumference), muscle strength (grip strength and the 5-times-sit-to-stand test) and physical performance (usual walking speed). In addition to investigating the microbiome composition, the study aims to elucidate microbiome functions by exploring potential omic associations with sarcopenia. To achieve this, salivary proteomics, metabolomics and quorum sensing peptidomics will be performed. Covariates that will be measured include clinical variables (sociodemographic factors, health status, health-related behaviours, oral health and quality of life) as well as blood variables (immune profiling, hormones, kidney and liver function, electrolytes and haematocrit). In phase 2, an in-depth mechanistic analysis will be performed on an envisaged subcohort of 50 participants. This analysis will explore pathways in muscle tissue using histology, genomics and transcriptomics, focusing on (maximal) 25 healthy older adults and (maximal) 25 with severe sarcopenia. Phase 3 involves a two-year clinical follow-up of the initial participants from the cross-sectional analysis, along with a resampling of blood and saliva. Additionally, secondary outcomes like falls, hospitalization and mortality will be examined.
DISCUSSION: Using a salivary multi-omics approach, SaMu primarily aims to clarify the associations between the oral microbiome and sarcopenia. SaMu is expected to contribute to the discovery of predictive biomarkers of sarcopenia as well as to the identification of potential novel targets to prevent/tackle sarcopenia. This study-protocol is submitted for registration at the ISRCTN registry.
Key words: Sarcopenia, muscle, saliva, microbiota, metabolomics, proteomics, quorum sensing peptides.
Abbreviations: QSP: quorum sensing peptides; QTOF: Quadrupole Time-of-Flight; MS: mass spectrometry; GC: gas chromatography; NMR: nuclear magnetic resonance; BIA: bio-electrical impedance; PSQI: Pittsburgh Sleep Quality Index; SarQOL: Sarcopenia – Quality of Life; PASE: Physical Activity Scale for the Elderly; DMFT: decayed, missing, filled teeth; DPSI: Dutch Periodontal Screening Index; MMSE: mini mental status examination; PBMC: peripheral blood mononuclear cell; CD: cluster of identification; PBMC: peripheral blood mononuclear cell; GEM: Genome Multitool.
Background
Sarcopenia, the accelerated loss of muscle mass, strength and function with ageing, is an important health challenge associated with decreased quality of life and increased mortality. Unfortunately the underlying pathophysiological mechanisms are very poorly understood (1). Evidence points towards a gut-muscle axis as part of the pathophysiology of sarcopenia, with short-chain fatty acids, infochemicals such as 2-amino acetophenone and quorum sensing peptides (QSP) being potential bacterial mediators (2-5).
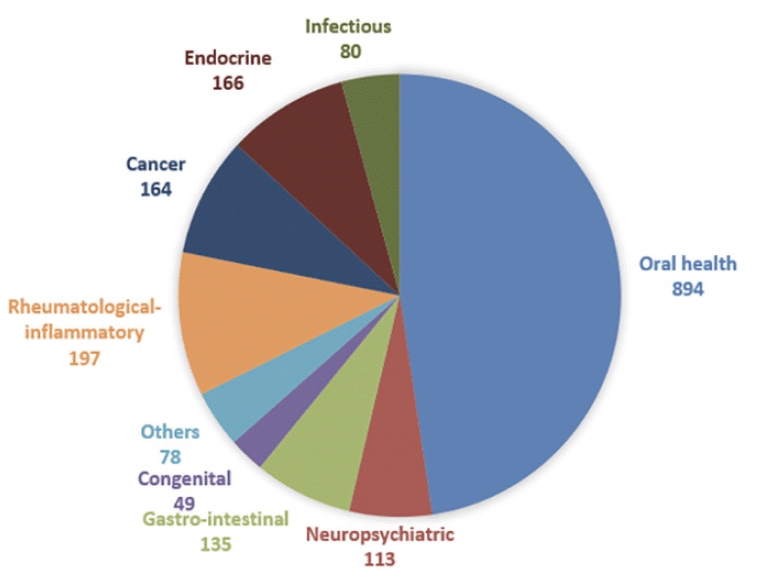
While host microbiome research in sarcopenia and other health conditions has traditionally focused on the gut microbiome, there is now growing interest in exploring other microbial ecosystems such as the oral microbiome. The oral cavity is, next to the gut, the compartment harbouring the highest abundance and diversity of microorganisms (6). The oral microbiome is associated with the progression of not only oral diseases such as dental caries, periodontal diseases, head and neck cancer, but also several systemic diseases such as cardiovascular disease, rheumatoid arthritis, adverse pregnancy outcomes, diabetes, lung infection, colorectal cancer, and pancreatic cancer (7, 8). In the Disbiome® database, as of April 2024, a total of 2023 studies have been identified linking the human oral microbiome to one of 78 different diseases (Figure 1). Oral health diseases such as periodontitis and caries account for almost 50% of these studies, followed by rheumatological-inflammatory diseases and cancer (9). Different niches can be identified in the oral ecosystem, i.e. some oral microbes adhere to the teeth, mucosa and tongue while others reside in the saliva. Saliva is a biofluid that shares microbiome with both dental and mucosal niches (10). Moreover, compounds originating from the different oral microbial niches, including proteins, peptides and small molecules, are present in the saliva, making this biofluid an interesting specimen rich of complex information.
Numbers are absolute amounts. A detailed overview of diseases can be found in Supplementary Table 1.
Very recently, some preliminary studies have been published investigating the association between salivary microbiota composition and frailty, a concept strongly associated with sarcopenia. Ogawa et al. compared nursing home residents, considered as the frail group, with community-living older adults, considered as the non-frail group, for salivary microbiota composition. In samples from the nursing home residents group, Actinomyces, Streptococcus, Bacilli, Selenomonas, Veillonella, and Haemophilus taxa showed higher relative abundance, while Prevotella, Leptotrichia, Campylobacter, and Fusobacterium had a lower relative abundance. A limitation in this study was the lack of diet adjustment in the analysis. Moreover, the bacterial information was limited by use of 16S rRNA amplicon sequencing, which can only identify most taxa up to the genus level or higher, i.e. no metagenomic nor strain information was available (11). Wells et al. found that frailty is associated with a decreased salivary microbiota diversity. They used the UK-Twin cohort data, adjusting for diet. However, they did not analyse which bacterial taxa are associated with frailty nor did they conduct shotgun metagenomics sequencing (12). In another recent study, Yang et al. demonstrated an association of dental caries with sarcopenia as well as with gut microbiota composition in an Asian community-living cohort aged 50 to 85 years (n=1442) (13). No study has yet investigated the salivary microbiome composition in association with sarcopenia.
Besides the microbial composition, investigating saliva metabolomics, proteomics or peptidomics in relation to sarcopenia could yield greater insights into the oral microbiome and identify potential mediators in the saliva matrix-muscle interactions. Some specific metabolites such as NO as well as inflammatory proteins such as IL-6 have been measured in the saliva and indirectly linked to frailty and/or sarcopenia (14, 15). In addition, untargeted saliva metabolomics in a young and aged group identified 99 metabolites, 21 of which were statistically different between the two age groups (16). All of the 21 metabolites declined in abundance with advancing age, except ATP, which increased 2-fold in the aged group, possibly due to reduced ATP consumption. Furthermore, research from our group has shown that QSP produced by oral bacteria can lead to muscle wasting in vitro as well as in mice (2, 17, 18). No study has yet investigated the salivary metabolomics, proteomics or peptidomics in association with frailty or sarcopenia.
The primary goal of this study is to identify a possible association between the oral microbiome, including the chemical composition of saliva, and sarcopenia.
Methods/Design
General
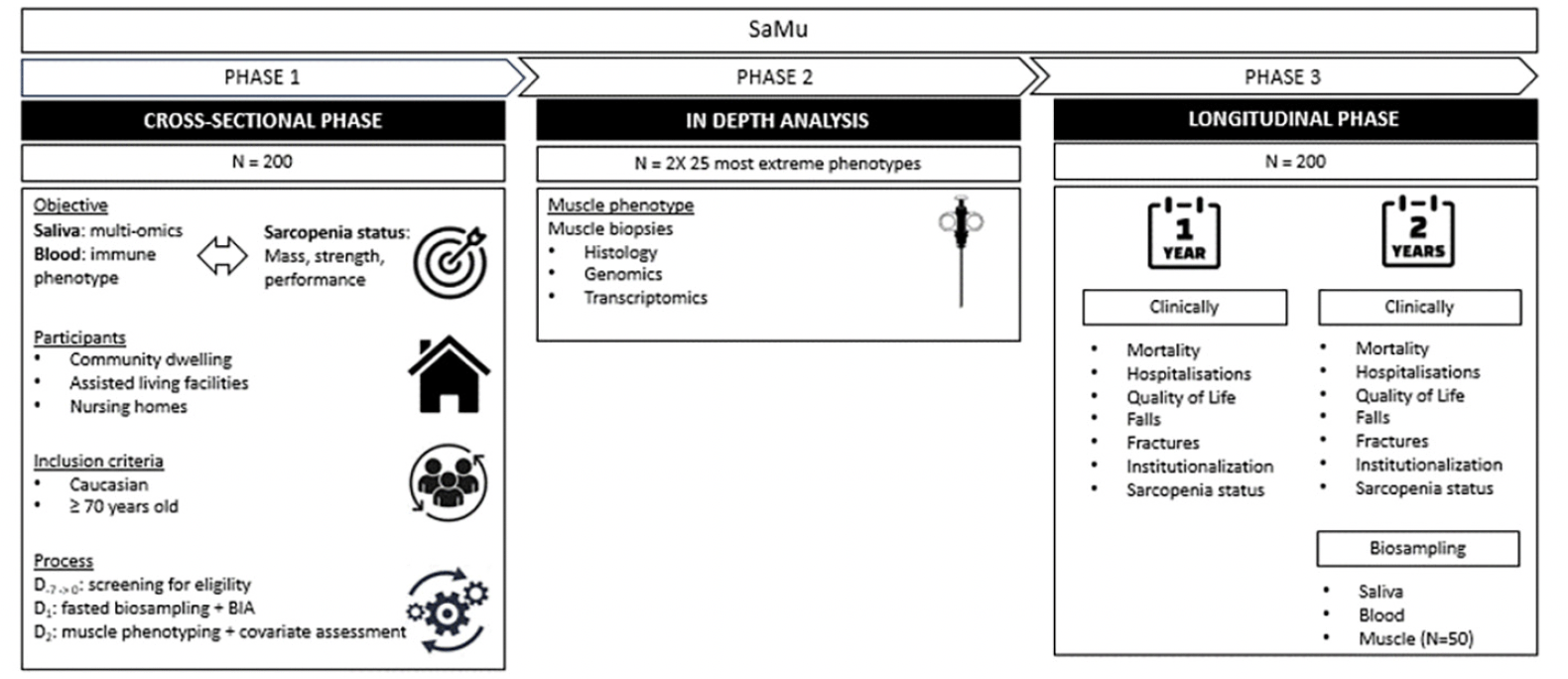
The SaMu study is coordinated by Ghent University hospital, in collaboration with the Ramen and Poel and Kanunnik Triest assisted living facilities and nursing homes in the city of Ghent, Belgium. Three phases can be distinguished. First the association between salivary multi-omics and sarcopenia status will be explored in 200 older people in a cross-sectional way. Phase 2 will focus on the most extreme phenotypes, with envisaged 50 participants—those in the healthiest and most sarcopenic quartiles—being selected from the final 100 participants of phase 1. These individuals will be asked to undergo additional muscle biopsies, which will be analyzed for histology, genomics, and transcriptomics. The third phase involves a two-year clinical follow-up of the initial participants from the cross-sectional analysis, along with a resampling of blood and saliva. Figure 2 provides a visual representation of the SaMu study design.
QSP= Quorum Sensing Peptides
Participants
A total of 200 Caucasian people aged 70 years or older will be recruited, without selection based on their level of sarcopenia or functional performance. To capture a continuous spectrum of sarcopenia, we will recruit a balanced sample from three key living environments that are associated with different levels of sarcopenia: community-dwelling individuals, those in assisted living facilities, and nursing home residents. Our goal is to achieve an approximately equal representation from each of these settings. Participants residing in assisted living facilities and nursing homes will be recruited from two prominent care homes in Ghent (Ramen and Poel and Kanunnik Triest), where all eligible residents will be individually informed about the study and invited to participate. Community-dwelling individuals will be recruited by identifying eligible participants attending outpatient services at Ghent University Hospital, as well as individuals participating in social activities organized in or near the two care homes. For the more invasive phase 2 of the SaMu study, participants from the last 100 of the cross-sectional phase who fall within the top or bottom 25% based on muscle score will be invited to also give a muscle biopsy. The muscle score quartiles will be determined using data from earlier participants. Letters informing the primary care physicians as well as closest family members of all participants about the SaMu study will be sent. Ethical approval has been granted by the Ghent University Hospital Ethics Committee (BC-1850).
Exclusion criteria are as follows: known presence of interfering neuromuscular or osteoskeletal conditions (possibly leading to false-positive diagnosis of sarcopenia), such as stroke without full recuperation, Parkinson’s disease, spinal compression, or functional anomaly of the hands or legs; >10% total body weight loss over the past 6 months; active malignant neoplasia; status post radiation therapy in the head-neck region; exposure to immunosuppressive drugs in the last 3 months before the screening visit; exposure to systemic corticosteroid treatment in the last 14 days before the screening visit; infection(s) requiring treatment with systemic antibiotics/antivirals/antifungals within 30 days prior to the biosampling; clinically detectable active infection (e.g. respiratory or gastro-intestinal). Finally, individuals who are not deemed competent to make decisions regarding their own well-being due to advanced cognitive impairment or severe psychiatric disease will not be included in the study.
Study procedures
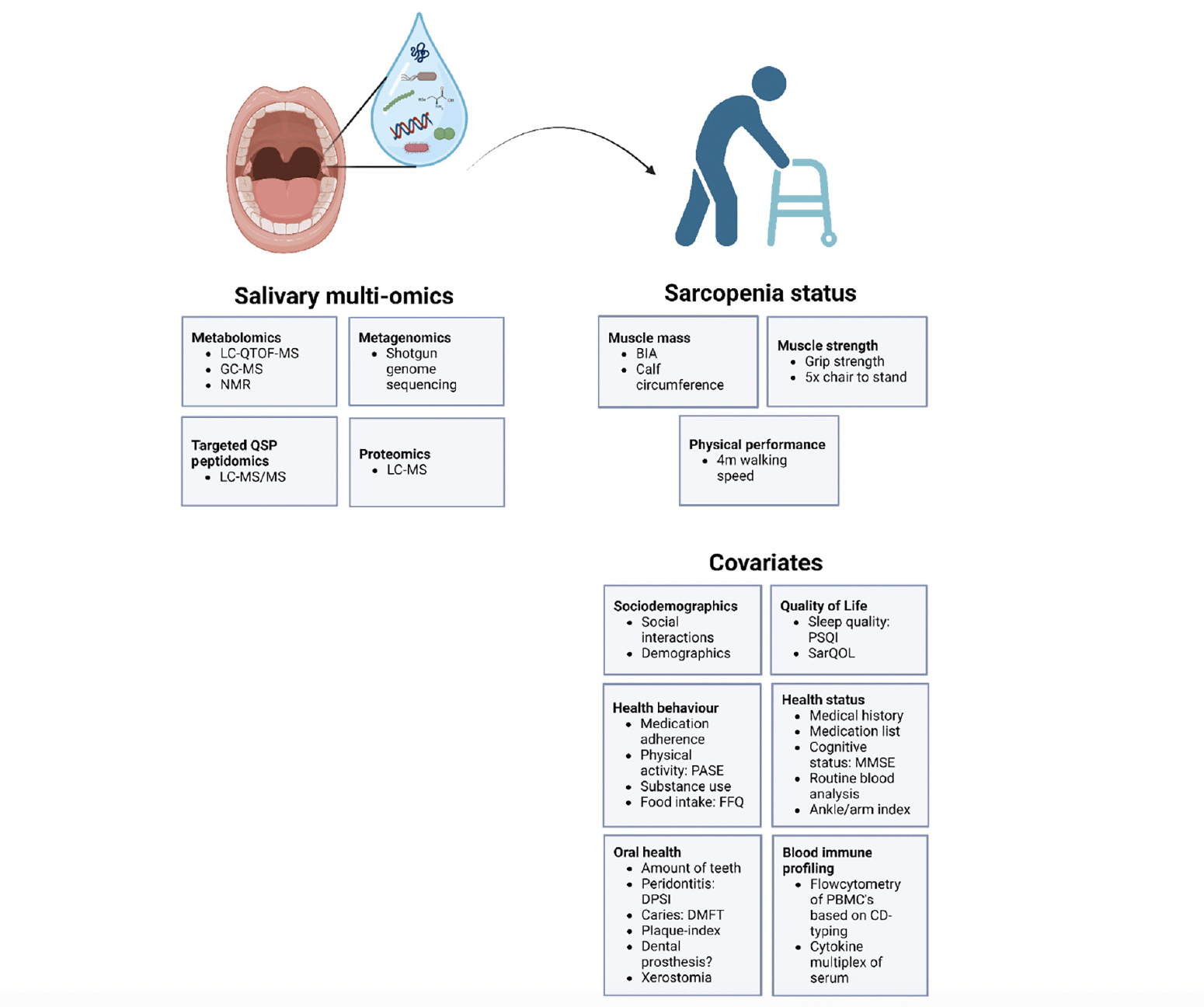
The SaMu study will be conducted in consecutive blocks, each comprising approximately 25-30 participants from different living arrangements. These blocks will run for one to two weeks, during which biosampling and clinical assessments will take place. Figure 3 provides an overview of the key variables measured. Recruitment, clinical characterization, sample collection, and analyses will occur within each block. Additionally, at least 2 young controls (aged 20-30 years) will be included in each block to act as internal controls, undergoing the same procedures as other participants. To prevent detection bias, researchers analyzing salivary multi-omics data will be blinded to all clinical measurements, including sarcopenia outcome variables, and vice versa (19).
QTOF= Quadrupole Time-of-Flight, MS= mass spectrometry, GC= gas chromatography, NMR= nuclear magnetic resonance, BIA= bio-electrical impedance, PSQI= Pittsburgh Sleep Quality Index, SarQOL= Sarcopenia – Quality of Life, PASE= Physical Activity Scale for the Elderly, DMFT= decayed, missing, filled teeth, DPSI= Dutch Periodontal Screening Index, MMSE= mini mental status examination, PBMC= peripheral blood mononuclear cell and CD= cluster of identification.
During the cross-sectional part, participants will undergo a series of four visits. Following an initial screening visit, participants will be visited on two consecutive mornings. On the first morning, participants will provide saliva and blood samples and undergo lean mass assessment in a fasted state (having fasted for at least 8 hours to ensure a standardized metabolic profile). In phase 2, muscle biopsies will be obtained from maximum 50 participants during this fasted morning or another fasted morning within one week of the blood and saliva sampling. On the second morning, muscle strength and function assessments, along with measurements of the clinical covariates, will be conducted following breakfast. Subsequently, one week after the conclusion of data collection, participants will receive their results, including their sarcopenia status and routine laboratory analyses, along with general recommendations regarding sarcopenia management. This information will also be communicated to their primary care physician.
For the prospective part, the sarcopenia status of participants, as well as secondary outcomes (mortality, hospitalisations, quality of life, falls, fractures, institutionalisation) will be assessed after one and two years. Furthermore, the salivary microbiome, covering various omics, along with a muscle biopsy from a maximum of n=50 participants, will undergo re-analysis after two years. This two-year full re-analysis represents a balance between anticipated clinical differences and cost-feasibility. Based on a twelve-year population study by Trevisan et al., we anticipate an overall mean clinical deterioration in sarcopenia status ranging from 15 to 50% after two years in the study population (20).
Screening visit (D-7→0)
SaMu begins with a screening visit during which the project will be explained, and written informed consent will be obtained by one of the researchers. A separate informed consent will be required for participation in the more invasive phase 2 and longitudinal phase 3, both of which are optional. Eligibility criteria will then be assessed using three sources. Firstly, the participant’s medical history and medication list will be reviewed, either directly with the participant or with the nurse in case of cognitive impairment. Subsequently, medical records will be examined for exclusion criteria by a medical doctor from the research team. Finally, if needed, the general physician or caregivers will be contacted for additional information. During the screening visit, eligible participants will also be instructed for the fasted morning visit, as outlined below.
Fasted morning visit: biosampling and BIA measurement (D1)
A detailed instruction form for the fasted morning visit is provided and explained to the participant during the screening visit. Eating or smoking is prohibited after midnight; only water is permitted. Participants should remove dentures overnight before sampling. The use of oral hygiene products, makeup, and medication is not permitted in the morning. Participants are encouraged to empty their bladder in the morning before BIA measurement. They should also limit physical activity and refrain from excessive talking during the morning. Finally, participants are instructed to drink a glass of water one hour before the fasted morning visit. During the fasted morning visit, researchers will verify compliance with the instructions and verify again the absence of the exclusion criteria, such as upper airway infections. Any deviations from the instructions will be carefully documented.
The fasted morning visit comprises four major steps: saliva collection, blood collection, BIA measurement and oral examination. Additionally, in phase 2, a fifth step will involve muscle tissue collection for a maximum of 50 participants.
Saliva collection and initial sample preparation
A 25 mL Protein LoBind tube will be stowed in a styrofoam cup filled with crushed ice. Participants will be instructed to rinse their mouth with tap water and minimize talking thereafter. Immediately following rinsing, bioelectrical impedance analysis (BIA), height, and weight measurements will be taken. Ten minutes after rinsing, participants will be asked to deliver as much saliva as possible into the 25 mL tube over a 5-minute period by spitting. Participants will be reminded not to cough up mucus and will be provided with gloves, as will the researcher assisting. The tube will then be sealed and returned to ice. Participants will be instructed to rinse their mouth again with tap water and wait an additional 10 minutes. During this interval, blood collection will take place. After 10 minutes, participants will again be asked to deliver saliva into the same cooled 25 mL tube for 5 minutes. Within 3 minutes of the final collection, the 25 mL tube will be centrifuged at 4000g for 5 minutes at 4°C. This process will precipitate large particles such as microbial and human epithelial cells, while retaining the chemical analytes in solution (21, 22). The saliva supernatant from each participant will be aliquoted (170 µL to 420 µL each, depending on analysis) into 1.5 mL Protein LoBind Eppendorf tubes, while the pellet will be dissolved in 1 mL phosphate-buffered saline (PBS). Both the supernatant aliquots and the cellular pellet will be immediately transferred to a tightly sealed container of dry ice within 5 minutes of centrifugation to ensure maximum stability. Salivary flow rate will be calculated by dividing the volume of collected saliva by the 10-minute collection period (23). A minimum of 2.5mL of fresh saliva is necessary for all analyses. Participants will be excluded from the study if the volume of saliva collected is inadequate.
Salivary analyses
To determine microbial composition, shotgun metagenomics sequencing will be conducted on the salivary pellet dissolved in PBS. Salivary supernatant aliquots will be used for general physico-chemical characterization (osmolality and total protein content) and five distinct omics analyses. Metabolomics will be assessed using LC-QTOF-MS , GC-MS and NMR. Proteomics and QSPeptidomics will be investigated using LC-MS/MS. Remaining aliquots will be stored at -80°C in the Ghent University Hospital biobank (FAGG-number BB190110).
Blood collection and initial sample preparation
Peripheral blood withdrawal will be conducted during the second 10-minute waiting period following mouth rinsing. Prior to collection, the blood draw site will be sterilized using a 70% isopropyl alcohol swab. For conventional blood analysis, the following tubes will be collected: 1x EDTA 4 mL; 2x serum 5 mL. Additionally, the following tubes will be collected for storage and subsequent analysis: 1x EDTA 10 mL; 3x heparin 10 mL; 1x serum 5 mL. The sequence for tube collection will remain consistent: 1) serum tubes, 2) EDTA tubes, and 3) heparin tubes. All tubes will be promptly mixed by inversion five times upon withdrawal. The three tubes designated for conventional blood analysis will be transported to Ghent University Hospital’s clinical laboratory within 3 hours of collection. The additional serum tube will be allowed to clot upright for 50 minutes at room temperature, then centrifuged at 1700g for 10 minutes at 22°C. The resulting supernatant (serum) will be stored in 1.5 mL protein LoBind Eppendorf tubes (in 500 µL aliquots) and transported on dry ice for storage at -80°C within 6 hours. The additional EDTA tube will be centrifuged at 1000g for 10 minutes at 4°C within 30 minutes after withdrawal. The plasma supernatant will be immediately transferred to 1.5 mL protein LoBind Eppendorf tubes (in 500 µL aliquots) on dry ice for storage at -80°C within 6 hours. Similarly, the additional heparin tubes will be centrifuged at 1700g for 10 minutes at 22°C within 30 minutes after withdrawal. A portion of the plasma supernatant will be promptly transferred to 1.5 mL protein LoBind Eppendorf tubes (in 500 µL aliquots) on dry ice for storage at -80°C, while peripheral blood mononuclear cells (PBMCs) will be isolated from the buffy coat layer using a Ficoll density centrifugation protocol within eight hours of blood collection.
Bio-electrical impedance (BIA) testing
Muscle mass will be evaluated using whole-body bioelectrical impedance analysis (BIA) (Bodystat Multiscan 5000, Euromedix, Leuven, Belgium). The participant (fasted state and post miction) must lie in a supine position with legs and arms not touching the body and with 2 surface electrodes placed on the right foot and hand, with a distance of 5 cm between both electrodes (distal electrode at metacarpophalangeal joints, proximal electrode at the level of radiocarpal joint). This test will not be conducted in case of a pacemaker or Implantable Cardioverter Defibrillator due to the risk of dysregulation of the device. To calculate the skeletal muscle mass (SM), the measured resistance and reactance values at 50 kHz will be imputed in the equation developed by Janssen et al. (24). This muscle mass will be adjusted for body height (25).
Oral examination
Following biosampling and BIA measurement, oral health will be evaluated by a dentist to establish a comprehensive oral health profile. The presence of tooth will be assessed, as bacterial diversity differs among dentulous and edentulous people (26). Additionally, the number of occluding pairs will provide insight into chewing ability. Various validated indices will also be utilized. The Dutch Periodontal Screening Index (DPSI), assessed using a dental probing instrument, will determine periodontal treatment needs (27). The DMFT index will evaluate the number of decayed teeth (= affected by active caries), missing teeth due to caries, and filled teeth (28). The Silness and Loë index will quantify plaque accumulation (29). Finally, participants will complete a validated questionnaire regarding xerostomia. Xerostomia will also be objectively assessed through salivary flow rate measurements.
Muscle tissue collection
For the skeletal muscle biopsies in phase 2, we will collect samples (200-300mg in total) from the vastus lateralis, utilizing the Bergström method with local anaesthetic. The obtained sample aliquots (approximately 25mg) will be promptly frozen in liquid nitrogen and stored at -80°C until further analyses. Using histology, genomics and transcriptomics, the following hallmarks of sarcopenia will be investigated: genomic instability (ratio of 8-hydroxy-2-deoxyguanosine to 2-deoxyguanosine), epigenetic alterations (DNA methylation patterns), loss of proteostasis (expression of key genes in the AKT/mTOR pathway), inflammation (expression of inflammatory genes such as CD14 and E2AK2), mitochondrial dysfunction (expression of genes involved in mitochondrial metabolism including the oxidative phosphorylation complex), cellular senescence (expression of cell cycle regulators such as p16 and p21), neural dysfunction (expression of neuromuscular genes such as Agrin), extracellular matrix dysfunction (collagen staining) and reduced vascular perfusion (endothelial staining). Additionally, histological analysis will involve immunohistochemical staining to identify and measure the cross-sectional areas of type I, IIa, and IIx muscle fibres.
Non-fasted morning visit: muscle phenotyping and covariate assessment (D2)
Muscle strength and physical performance measurements, along with the assessment of clinical covariates (excluding oral examination), will be conducted during the non-fasted morning visit. This session occurs after participants have had breakfast, on the day following the fasted morning visit.
Muscle mass testing
In addition to fasting BIA measurements, bilateral calf circumference is also assessed, in a seated position. While this method possesses lower discriminatory capability and may be influenced by factors such as oedema, it is a validated proxy for muscle mass (30).
Muscle strength testing
Grip strength is evaluated using a Jamar+ Digital Dynamometer (Patterson Medical, Warrenville, IL, USA) following the Southampton protocol (31). Participants hold the dynamometer in a 90° elbow joint angle and squeeze with maximal effort, while the forearm rests on the armchair. The investigator gently supports the dynamometer in an upright position. They perform three attempts per arm, alternating left-right, and the maximal value (in kg) from the six tries is used for analysis.
Also a 5-times-sit-to-stand test is performed. An armchair is placed with backrest against the wall. Both hands are placed at the contralateral shoulder. Legs are positioned in a 90° angle with trunk. Then participants are asked to stand (knees extended maximally) and sit five times as fast as possible. The examiner first shows twice how to perform the test. Afterwards, the participant can try twice. Time starts when participant raises the first time and stops right after the participant is seated the fifth time.
Physical performance
To evaluate physical performance, patients are asked to walk (at a normal pace) along a straight line 6 m long in distance. The time taken to walk the middle 4 m (excluding 1 m accelerating and 1 m decelerating phase), is subsequently used to calculate the usual walking speed. Participants using a walking aid (walker or cane) during daily activities (to walk inside the house), also use this during the test. Time to walk 4 meters is measured twice. Exceptionally, a third measurement is performed when the two first values differ too much (> 20%). The mean value is used in further analysis.
Covariates
Microbial composition is influenced by various factors such as age, gender and the exposome, defined as «the cumulative measure of environmental influences and associated biological responses throughout the lifespan, including exposures from the environment, diet, behaviour, and endogenous processes» (32). To address these factors in the SaMu cohort, a comprehensive assessment involving thorough medical history, clinical examination, and completion of questionnaires will be conducted. Figure 3 provides an overview of all covariates considered.
In general, the SaMu covariates can be categorized into six groups. First, sociodemographics encompass social interactions, including family and friend contacts, as well as membership in cultural or sports associations. Basic demographics such as age, sex, residential history (especially rural or urban environments), previous occupation, marital status, and socioeconomic status will also be considered. Secondly, quality of life (QOL) will be explored, focusing on sarcopenia-related QOL and sleep quality through the completion of two validated questionnaires. The Pittsburgh Sleep Quality Index is validated for assessing subjective sleep quality in older adults (33), while the Sarcopenia and Quality of Life (SarQoL) questionnaire evaluates health-related QOL specific to sarcopenia (34). Third, health status evaluation will involve reviewing medical history and medications as well as a routine blood analysis including testosterone, vitamine D, thyroid hormones, kidney and liver function, electrolytes and haematocrit. An ankle-arm index will screen for peripheral vascular disease. Cognitive status will be assessed using the MMSE. Fourth, health behaviour will be investigated through assessments of medication adherence and substance use. Diet quality will be evaluated using the short version of a Food Frequency Questionnaire, validated for older adults (35). Additionally, the Physical Activity Scale for the Elderly (PASE) will measure physical activity levels in this population (36). Fifth, oral health, as detailed earlier, significantly influences both oral microbiome and overall health. Sixth, blood immune phenotyping will be assessed using flow cytometry on peripheral blood mononuclear cells (PBMCs). This includes assessing various parameters such as the number of B and T cells, the ratio of naïve to memory T cells, and the quantity of innate-like T cells. Additionally, serum cytokine levels will be measured to explore four key inflammatory pathways: innate immune activation (e.g. IL-15 and MCP-1), systemic inflammation (e.g. IL-6 and IL-8), immune regulation (e.g. PDL-1 and IL-4), and endothelial function (e.g. TGF-α and ICAM-1).
Data analysis
Data processing
During the recruitment process, a unique ID number will be assigned to each participant by the responsible clinical researcher (pseudonymization). Lab researchers who handle and analyze biosamples, will only receive this ID number along with the samples, ensuring they remain blinded to any clinical information throughout the study. Furthermore, all clinical data will be securely stored on a system provided by Ghent University and will be de-identified to meet the HIPAA standards for a ‘Limited Data Set’ (which excludes the 16 defined identifiers under HIPAA). Raw data, partially processed data, and fully processed data from both clinical procedures and biosampling will be sent from individual labs to a central registry. Access to this central registry will be restricted to researchers responsible for data management (different from those conducting the clinical and lab analyses) and bioinformaticians.
For shotgun metagenomics sequencing data, after demultiplexing and an Illumina internal quality filtering procedure called chastity filter, FASTQ files will be collected. Further processing will consist of filtering metagenomic reads containing Illumina adapters and low-quality reads, and trimming low-quality read edges. Human reads will be identified and removed by mapping the reads to the human genome (hg38) with the Genome Multitool (GEM) mapper (37). Tables of relative abundance estimates of bacterial taxa will be constructed using both marker-gene (38) and k-mer (39) based approaches; downstream analyses will be performed on both outputs and then compared for consistency of results. Filters will be applied to each abundance table, to remove rare taxa using criteria as previously described (40). For analysis of LC-MS and GC-MS metabolomics data, first pre-processing to extract ions from raw data will take place. For LC-MS, raw data in Agilent will be in ‘.d’ format, and for GC-MS ‘.D’ format will be handled. Further data processing to assure data quality and transform raw values will be performed. For NMR analysis, the 1H-NMR spectra will be analysed using two procedures: intelligent bucketing and manual deconvolution. With the first procedure, the values of the relative integrals of about 100 regions of the spectrum will be obtained for each sample. Meanwhile, with the deconvolution procedure, the absolute concentrations of about 50 metabolites present in the saliva of the subjects enrolled in the study will be obtained. Both these procedures will lead to constructing two matrices, constituting the NMR raw data. Furthermore, similar methods used for LC-MS and GC-MS, along with normalization strategies based on osmolality values, will be applied to NMR data processing. For QSPeptidomics, QSP signal intensities constitute the raw data, with data-processing similar to LC-MS metabolomics. The raw data of the proteomics analysis (MS intensities) will be processed using the DiaNN algorithm, and spectra will be matched against the human protein sequences in the Swiss-Prot database (41).
The sarcopenia status will be a quantitative composite measurement of muscle mass measured through BIA, muscle strength measured through grip strength and physical performance measured through walking speed (42). For sensitivity analyses and population comparisons with populations described in the literature, muscle mass and muscle strength will also be measured through calf circumference and 5-times-sit-to-stand test respectively. Calf circumference has less discriminating power compared to BIA muscle mass measurements (43), while the 5-times-sit-to-stand test is more dependent on ‘acute’ changes (e.g. balances shifts with disease) and hence an inferior estimate for chronic sarcopenia (44). Sarcopenia status is expressed both categorically based on the EWGSOPII guidelines and continuously based on desirability functions (42, 45). To integrate the different continuous variables into one physiologically relevant continuous response, the multi-criteria Derringer’s concept of desirability is used. Every sarcopenia response (i.e. muscle mass, grip strength and physical performance) is first linearly transformed into a dimensionless desirability (d) value, ranging from 0.1 to 0.9, where 0.1 and 0.9 are the lowest and highest obtained value of that response amongst all patients respectively. If participants are unable to walk (too weak), the walking speed is set to 0.05 m/s. If participants are unable to perform the 5-times-sit-to-stand test, time is set to 60 seconds. Both are translated to a d-value of 0.1 in this model. These three standardized d-values are combined into a global D-value for each patient, which is the geometric mean (robust for outliers; missing values are not imputed). Each component (muscle mass, muscle strength and physical performance) is weighed equally. Inherent to this desirability concept, all D-values lie between 0.1 and 0.9, with 0.1 indicating a relatively high sarcopenic status (low muscle desirability) and 0.9 a relatively low sarcopenic status (high muscle desirability). If one or two components are missing (e.g. if muscle mass cannot be assessed via BIA due to the presence of a pacemaker), the global D-value will still be calculated using the geometric mean of the available components, without imputation of missing values.
Statistical analyses
To identify the microbial taxa and other analytes (including metabolites, proteins and QSP) that significantly differ between sarcopenic and healthy older adults, we will apply both univariate and predictive modelling approaches, with consistency comparisons performed to infer robust results as previously described (40). For each sub-analysis, we will use the intersection of samples and variables that are available such that we do not have missing data for that analysis. In cases in which we have particular patterns of missing data related to measurement limitations (i.e. assays with limits of detection), we will use appropriate statistical modeling approaches to model true zeros vs. low quantities. Given that drug intake can have a large impact on the host microbiota (46, 47), we will also perform analyses to identify and filter out microbiome and metabolome features from downstream analyses that are strongly associated with drug usage (48). For univariate analyses of counts-based data, we will use DESeq2 modelling (49). For univariate analyses of continuous-valued variables, we will use the two-sided Mann-Whitney U-test. In both cases, multiple hypotheses corrections will be performed using the method of Benjamini and Hochberg (50). Predictive analyses will be performed using a cross-validation framework as previously described (40). Briefly, microbial taxa and analytes will serve as features for machine learning methods (e.g., lasso logistic regression, Random Forests and deep learning approaches) with the task of prediction of binary or continuous valued sarcopenia status. Relevant clinical covariates, as previously described, will also be included in the models. Predictive performance of models will be assessed as cross-validated F1 scores and area under the receiver operator curve (AUC) for binary values, and root mean-squared-error for continuous values. Features yielding the highest cross-validated accuracies will be tabulated and assessed for consistency across methods, to establish sets of biomarkers of sarcopenia status. We will also assess secondary outcomes in these analyses, including falls, fractures, quality of life, hospitalization, institutionalization and mortality.
Discussion
SaMu aims to clarify the associations between the oral microbiome and sarcopenia by a three-fold process. First, cross-sectionally, by exploring the salivary bacterial composition including their analytes in relationship to sarcopenia in 200 older people. Secondly, there will be a parallel focus on in-depth mechanistic analysis of a subcohort consisting of the most extreme phenotypes. Thereto, the relative contribution of different sarcopenia hallmarks in microbiome-sarcopenia associations will be investigated. Finally, prospective follow-up of all participants will take place, both clinically including sarcopenia status and adverse outcomes after 1 year and 2 years, and biochemically including metabolomics, proteomics, QSPeptidomics, metagenome sequencing and muscular histology, genomics and transcriptomics after 2 years.
The first strength of this study is its holistic design, both for predictor variables, outcome variables and covariates. The analysis of different omics in parallel, together with shotgun genomic sequencing of bacterial DNA allows for sophisticated analyses of the complex interplay of factors. Also, the primary outcome variable of SaMu will be an integrated sarcopenia assessment, going broader than muscle mass, strength or function assessment alone, as well as including mechanistical markers using muscle tissue biopsies. Finally, covariates will cover a broad range of different exposome dimensions. Such a comprehensive approach has not yet been applied in the context of the oral microbiota and sarcopenia.
Secondly, the use of state-of-the art validated -omics methods is an important asset. QSPeptidomics will be analysed by optimized LC-MS/MS. Additionally, metabolomics will be assessed through NMR, LC-MS, and GC-MS analyses, while proteomics will be conducted via LC-MS. Finally, shotgun metagenomics sequencing will be employed to identify bacterial composition.
Thirdly, conducting all clinical tests, biosampling, and initial sample preparations bedside ensures high participant feasibility and minimizes dropout bias. This approach enables the inclusion of a unique population, including very dependent older individuals, while also ensuring the highest sample stability, which is crucial for peptidomics analysis (51).
Fourthly, despite the study’s associative nature, the mechanistic and longitudinal components, combined with the holistic approach and the thorough exposure-outcome blinding, will serve exploratory purposes in understanding causality.
Some limitations of the SaMu study should be considered. Firstly, because all care home participants will be recruited from only 2 centers in the same city, Ramen and Poel and Kanunnik Triest, SaMu may be susceptible to selection bias. Given the diverse population at the both centers, and anticipated high participation rates, we expect this bias to be minimal; moreover the reduced heterogeneity within a city or care center may lower the likelihood of unobserved confounding related to place of living. However, we recognize that findings will need to be validated in more diverse populations to fully assess generalizability. Additionally, due to the exploratory nature of SaMu, no sample size calculations were conducted, but it aligns with or exceeds that of previous similar studies (52-54). Third, while BIA will be used to assess muscle mass instead of dual-energy X-ray absorptiometry (DXA) or magnetic resonance imaging (MRI) — which offer greater precision — BIA has been well-validated and ensures high feasibility, especially in frail older adults, due to its portability and ease of use. Strict criteria for BIA conditions (e.g. fasted state, post-micturition, supine position) will be followed to minimize precision loss. Fourth, individual results from the cross-sectional assessment (physical tests and routine lab results), will be shared with participants and their primary care physicians, along with general advice on sarcopenia management. While this could potentially introduce bias, we will monitor changes in food intake, medication, and physical activity over the 1-year and 2-year follow-ups. This will allow us to adjust for any interventions and minimize potential bias in the longitudinal analysis. Lastly, the inclusion of very frail individuals may pose challenges in interpreting pre-sampling instructions accurately. To address this, comprehensive explanations will be provided to all nurses and caregivers, who will assist participants in following instructions.
Studying the relationship between salivary microbiota and its analytes, and sarcopenia has significant diagnostic and therapeutic implications. Predictive biomarkers that can anticipate the progression of sarcopenia can help to identify those at the highest risk. This method presents numerous advantages over traditional plasma approaches, including cost reduction, non-invasive and stress-free specimen collection, and safer and simpler sample collection, which even allows for self-collection. As a result, obtaining repeated specimens is not challenging. Saliva has been recognized by scientists as a comprehensive indicator of health, containing a complete range of proteins, hormones, antibodies, and other analytes commonly measured in blood tests (55). For example, 20-30% of proteins found in saliva are also found in blood, according to comparisons between saliva and plasma proteomes (56). Additionally, discovering new salivary analytes, such as quorum sensing peptides, in association with sarcopenia, can help to identify potential causative factors in sarcopenia’s pathophysiology and could be evaluated in preclinical causal studies.
Ethics approval and consent to participate: Ethical approval has been granted by the Ghent University Hospital Ethics Committee (BC-1850). Written informed consent will be obtained from all participants. A separate informed consent will be required for participation in the more invasive phase 2 and longitudinal phase 3, both of which are optional.
Consent for publication: Not applicable.
Availability of data and materials: Not applicable.
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding: This work and ADS are supported by a FIKO (Fonds voor Innovatie en Klinisch Onderzoek) grant from the University Hospital Ghent. LC is supported by a grant of Research Foundation – Flanders (FWO) (grant number 1S53323N).
Authors’ contributions: ADS conceived and designed the study together with EW and BDS. LC, ADS, EW and BDS, drafted and revised the article. LC, ML, JC, DE, CVDL, PC, NVDN, BDS, EW and ADS were involved in the design of the clinical parts and revised the article. AD, HH, DS, MM, MB, GP, DC, SC, TS, GKG, CT, and PN were involved in the design of the bio-analytical parts and revised the article. All authors gave final approval of the manuscript to be published.
Acknowledgements: Not applicable.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Wiedmer, P. et al. Sarcopenia – Molecular mechanisms and open questions. Ageing Research Reviews 65, 101200 (2021). https://doi.org/10.1016/j.arr.2020.101200
2 De Spiegeleer, A. et al. Quorum sensing molecules as a novel microbial factor impacting muscle cells. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1866, 165646 (2020). https://doi.org/10.1016/j.bbadis.2019.165646
3 Tzika, A. A. et al. A Small Volatile Bacterial Molecule Triggers Mitochondrial Dysfunction in Murine Skeletal Muscle. Plos One 8, e74528 (2013). https://doi.org/10.1371/journal.pone.0074528
4 Zhang, T., Cheng, J. K. & Hu, Y. M. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Research Reviews 81, 101739 (2022). https://doi.org/10.1016/j.arr.2022.101739
5 De Spiegeleer, A. et al. The bacterial quorum sensing peptide iAM373 is a novel inducer of sarcopenia. Clinical and Translational Medicine 12, e1053 (2022). https://doi.org/10.1002/ctm2.1053
6 Verma, D., Garg, P. K. & Dubey, A. K. Insights into the human oral microbiome. Archives of Microbiology 200, 525-540 (2018). https://doi.org/10.1007/s00203-018-1505-3
7 Peng, X. et al. Oral microbiota in human systematic diseases. International Journal of Oral Science 14, 14 (2022). https://doi.org/10.1038/s41368-022-00163-7
8 Sekundo, C., Langowski, E., Wolff, D., Boutin, S. & Frese, C. Maintaining oral health for a hundred years and more?-An analysis of microbial and salivary factors in a cohort of centenarians. Journal of Oral Microbiology 14, 2059891 (2022). https://doi.org/10.1080/20002297.2022.2059891
9 Janssens, Y. et al. Disbiome database: linking the microbiome to disease. Bmc Microbiology 18, 50 (2018). https://doi.org/10.1186/s12866-018-1197-5
10 Xu, X. et al. Oral cavity contains distinct niches with dynamic microbial communities. Environmental Microbiology 17, 699-710 (2015). https://doi.org/10.1111/1462-2920.12502
11 Ogawa, T. et al. Composition of salivary microbiota in elderly subjects. Scientific Reports 8, 414 (2018). https://doi.org/10.1038/s41598-017-18677-0
12 Wells, P. M. et al. Influential factors of saliva microbiota composition. Scientific Reports 12, 18894 (2022). https://doi.org/10.1038/s41598-022-23266-x
13 Yang, Y. et al. Association of Dental Caries with Muscle Mass, Muscle Strength, and Sarcopenia: A Community-Based Study. Journal of Nutrition Health & Aging 27, 10-20 (2023). https://doi.org/10.1007/s12603-022-1875-8
14 Gomez-Rubio, P., Trapero, I., Cauli, O. & Buigues, C. Salivary IL-6 Concentration Is Associated with Frailty Syndrome in Older Individuals. Diagnostics 12, 117 (2022). https://doi.org/10.3390/diagnostics12010117
15 Zhurakivska, K. et al. Do Changes in Oral Microbiota Correlate With Plasma Nitrite Response? A Systematic Review. Frontiers in Physiology 10 (2019). https://doi.org/10.3389/fphys.2019.01029
16 Teruya, T., Goga, H. & Yanagida, M. Human age-declined saliva metabolic markers determined by LC-MS. Scientific Reports 11, 18135 (2021). https://doi.org/10.1038/s41598-021-97623-7
17 De Spiegeleer, A. et al. Streptococcal quorum sensing peptide CSP-7 contributes to muscle inflammation and wasting. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1870 (2024). https://doi.org/10.1016/j.bbadis.2024.167094
18 Verbeke, F. et al. A fit-for-purpose LC-MS/MS method for the analysis of selected Streptococcal quorum sensing peptides in human saliva. J Pharm Biomed Anal 213, 114594 (2022). https://doi.org/10.1016/j.jpba.2022.114594
19 Parker, R. A. in Planning clinical research Ch. Blinding in observationel studies, (Cambridge University press, 2016).
20 Trevisan, C., Vetrano, D. L., Calvani, R., Picca, A. & Welmer, A. K. Twelve-year sarcopenia trajectories in older adults: results from a population-based study. Journal of Cachexia Sarcopenia and Muscle 13, 254-263 (2022). https://doi.org/10.1002/jcsm.12875
21 Schipper, R. et al. SELDI-TOF-MS of saliva: Methodology and pre-treatment effects. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 847, 45-53 (2007). https://doi.org/10.1016/j.jchromb.2006.10.005
22 Barranco, T. et al. Impact of Saliva Collection and Processing Methods on Aspartate Aminotransferase, Creatin Kinase and Lactate Dehydrogenase Activities. Analytical Sciences 34, 619-622 (2018). https://doi.org/10.2116/analsci.17N035
23 Machida, T. et al. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. International Journal of Molecular Sciences 16, 21294-21309 (2015). https://doi.org/10.3390/ijms160921294
24 Janssen, I., Heymsfield, S. B., Baumgartner, R. N. & Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. Journal of Applied Physiology 89, 465-471 (2000). https://doi.org/10.1152/jappl.2000.89.2.465
25 Han, D. S. et al. Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Scientific Reports 6, 19457 (2016). https://doi.org/10.1038/srep19457
26 Gazdeck, R. K. et al. Diversity of the oral microbiome between dentate and edentulous individuals. Oral Diseases 25, 911-918 (2019). https://doi.org/https://doi.org/10.1111/odi.13039
27 Van der Velden, U. The Dutch periodontal screening index validation and its application in The Netherlands. Journal of Clinical Periodontology 36, 1018-1024 (2009). https://doi.org/https://doi.org/10.1111/j.1600-051X.2009.01495.x
28 van der Heijden, E. M., Klüter, W. J., van der Maarel-Wierink, C. D. & Gobbens, R. J. J. Exploring associations between multidimensional frailty and oral health in community-dwelling older people. A pilot study. Special Care in Dentistry 42, 361-368 (2022). https://doi.org/https://doi.org/10.1111/scd.12691
29 Pretty, I. A., Edgar, W. M., Smith, P. W. & Higham, S. M. Quantification of dental plaque in the research environment. Journal of Dentistry 33, 193-207 (2005). https://doi.org/https://doi.org/10.1016/j.jdent.2004.10.017
30 Kiss, C. M. et al. Calf circumference as a surrogate indicator for detecting low muscle mass in hospitalized geriatric patients. Aging Clinical and Experimental Research 36, 25 (2024). https://doi.org/10.1007/s40520-024-02694-x
31 Roberts, H. C. et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40, 423-429 (2011). https://doi.org/10.1093/ageing/afr051
32 Miller, G. W. & Jones, D. P. The Nature of Nurture: Refining the Definition of the Exposome. Toxicological Sciences 137, 1-2 (2013). https://doi.org/10.1093/toxsci/kft251
33 Buysse, D. J. et al. Quantification of Subjective Sleep Quality in Healthy Elderly Men and Women Using the Pittsburgh Sleep Quality Index (PSQI). Sleep 14, 331-338 (1991). https://doi.org/10.1093/sleep/14.4.331
34 Beaudart, C. et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. Journal of Cachexia Sarcopenia and Muscle 8, 238-244 (2017). https://doi.org/10.1002/jcsm.12149
35 Robinson, S. M. et al. Development of a short questionnaire to assess diet quality among older community-dwelling adults. The journal of nutrition, health & aging 21, 247-253 (2017). https://doi.org/10.1007/s12603-016-0758-2
36 Washburn, R. A., Smith, K. W., Jette, A. M. & Janney, C. A. The physical activity scale for the elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology 46, 153-162 (1993). https://doi.org/https://doi.org/10.1016/0895-4356(93)90053-4
37 Marco-Sola, S., Sammeth, M., Guigó, R. & Ribeca, P. The GEM mapper: fast, accurate and versatile alignment by filtration. Nature Methods 9, 1185-U1176 (2012). https://doi.org/10.1038/nmeth.2221
38 Blanco-Míguez, A. et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nature Biotechnology 41, 1633-1644 (2023). https://doi.org/10.1038/s41587-023-01688-w
39 Lu, J. et al. Metagenome analysis using the Kraken software suite. Nature Protocols 17, 2815-2839 (2022). https://doi.org/10.1038/s41596-022-00738-y
40 Dawkins, J. J. et al. Gut metabolites predict Clostridioides difficile recurrence. Microbiome 10, 87 (2022). https://doi.org/10.1186/s40168-022-01284-1
41 Demichev, V., Messner, C. B., Vernardis, S. I., Lilley, K. S. & Ralser, M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nature Methods 17, 41–44 (2020). https://doi.org/10.1038/s41592-019-0638-x
42 De Spiegeleer, A. et al. Acute sarcopenia changes following hospitalization: influence of pre-admission care dependency level. Age and Ageing 50, 2140-2146 (2021). https://doi.org/10.1093/ageing/afab163
43 Quiñonez-Olivas, C. G. et al. Muscle mass measured using bioelectrical impedance analysis, calf circumference and grip strength in older adults. Medicina Universitaria 18, 158-162 (2016). https://doi.org/10.1016/j.rmu.2016.06.005
44 Verstraeten, L. M. G. et al. Handgrip strength rather than chair stand test should be used to diagnose sarcopenia in geriatric rehabilitation inpatients: REStORing health of acutely unwell adulTs (RESORT). Age and Ageing 51, afac242 (2022). https://doi.org/10.1093/ageing/afac242
45 Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing 48, 16-31 (2019). https://doi.org/10.1093/ageing/afy169
46 DeClercq, V., Nearing, J. T. & Langille, M. G. I. Investigation of the impact of commonly used medications on the oral microbiome of individuals living without major chronic conditions. Plos One 16, e0261032 (2021). https://doi.org/10.1371/journal.pone.0261032
47 Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623-628 (2018). https://doi.org/10.1038/nature25979
48 Forslund, S. K. et al. Combinatorial, additive and dose-dependent drug-microbiome associations. Nature 600, 500-505 (2021). https://doi.org/10.1038/s41586-021-04177-9
49 Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550 (2014). https://doi.org/10.1186/s13059-014-0550-8
50 Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 57, 289-300 (1995). https://doi.org/https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
51 Debunne, N. et al. Influence of Blood Collection Methods and Long-Term Plasma Storage on Quorum-Sensing Peptide Stability. Acs Omega 5, 16120-16127 (2020). https://doi.org/10.1021/acsomega.0c01723
52 Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nature Medicine 21, 895-905 (2015). https://doi.org/10.1038/nm.3914
53 Feng, Q. et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nature Communications 6, 6528 (2015). https://doi.org/10.1038/ncomms7528
54 Huang, K. et al. Salivary Microbiota for Gastric Cancer Prediction: An Exploratory Study. Frontiers in Cellular and Infection Microbiology 11 (2021). https://doi.org/10.3389/fcimb.2021.640309
55 Niebla-Cardenas, A. et al. Translational research into frailty from bench to bedside: Salivary biomarkers for inflammaging. Experimental Gerontology 171, 112040 (2023). https://doi.org/10.1016/j.exger.2022.112040
56 Yan, W. H. et al. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clinical Applications 3, 116-134 (2009). https://doi.org/10.1002/prca.200800140
The Author(s) 2024