T. Zanotto1,2,3,4, T.H. Mercer4, A. Gupta5, M.L. van der Linden4, P. Koufaki4
1. Department of Occupational Therapy Education, School of Health Professions, University of Kansas Medical Center, Kansas City, KS, United States; 2. Mobility Core, University of Kansas Center for Community Access, Rehabilitation Research, Education and Service, Kansas City, KS, United States; 3. Landon Center on Aging, University of Kansas Medical Center, Kansas City, KS, United States; 4. Centre for Health, Activity and Rehabilitation Research, School of Health Sciences, Queen Margaret University, Edinburgh, United Kingdom; 5. Division of Nephrology and Hypertension and the Jared Grantham Kidney Institute, University of Kansas Medical Center, Kansas City, KS, United States.
Corresponding Author: Tobia Zanotto, Ph.D., Department of Occupational Therapy Education, School of Health Professions, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS, 66160, United States. Email: tzanotto@kumc.edu
J Frailty Aging 2024;13(4)534-540
Published online August 10, 2024, http://dx.doi.org/10.14283/jfa.2024.61
Abstract
BACKGROUND: High blood pressure variability (BPV) is a predictor of cardiovascular events and all-cause mortality in people with end-stage kidney disease (ESKD) and a marker of aging in geriatric populations. Nevertheless, the relationship between BPV and geriatric syndromes, such as frailty, in people with ESKD is not well understood.
OBJECTIVE: To examine the association between very short-term BPV and frailty in people with ESKD and receiving hemodialysis.
DESIGN: Cross-sectional study.
SETTING: Three dialysis units in the United Kingdom.
PARTICIPANTS: Sixty-nine people receiving hemodialysis (median age=62.0 years, interquartile range [IQR]=19.0; 52.2% male; median dialysis vintage=1.1 years, IQR=2.4).
MEASUREMENTS: Systolic and diastolic BPV were recorded using continuous, non-invasive BP monitoring (Task Force Monitor). The very low, low, and high frequency components of BPV (VLF-BPV, LF-BPV, and HF-BPV), as well as the power spectral density (PSD-BPV) and low frequency/high frequency ratio of BPV (LF/HF-BPV) were analyzed. Frailty was evaluated using the Fried frailty phenotype.
RESULTS: Twenty-six (37.7%) participants were classified as frail and 43 (62.3%) as non-frail. Frail participants had higher median systolic (2.1, IQR=5.2 mmHg2 vs. 1.1, IQR=1.6 mmHg2, p=0.002) and diastolic HF-BPV (0.9, IQR=2.3 mmHg2 vs. 0.5, IQR=1.0 mmHg2, p=0.048) compared to their non-frail counterparts. In addition, frail participants had higher median systolic VLF-BPV (3.2, IQR=12.5 mmHg2 vs. 2.0, IQR=2.4 mmHg2, p=0.012), LF-BPV (2.0, IQR=3.8 mmHg2 vs. 1.1, IQR=2.0 mmHg2, p=0.016), and PSD-BPV (6.6, IQR=27.6 mmHg2 vs. 4.5, IQR=5.9 mmHg2, p=0.005) compared to the non-frail participants. In age- and sex-adjusted logistic regression analyses, only systolic VLF-BPV (odds ratio [OR]=1.13, 95% confidence interval [CI]:1.01-1.26, p=0.035), HF-BPV (OR=1.26, 95%CI:1.01-1.57, p=0.044), and PSD-BPV (OR=1.06, 95%CI:1.01-1.12, p=0.029) were associated with increased odds of being frail.
CONCLUSION: Higher systolic BPV is associated with frailty in people receiving hemodialysis. Beat-to-beat assessments of BPV through continuous, non-invasive BP monitoring may be useful in evaluating frailty in ESKD populations.
Key words: Frailty, cardiovascular diseases, end-stage kidney disease, blood pressure.
Introduction
Cardiovascular disease and frailty represent two major clinical concerns for people living with end-stage kidney disease (ESKD) (1, 2). Cardiovascular disease affects approximately 50% of people living with advanced chronic kidney disease (stages 4-5) and is the leading cause of death in people with ESKD, accounting for 40-50% of deaths (3). In addition, systematic reviews with meta-analysis have concluded that about 36% of people receiving hemodialysis (HD) for ESKD meet objective diagnostic criteria for frailty (4, 5). Both cardiovascular disease and frailty are strongly associated with adverse clinical outcomes, such as falls, hospitalizations, and all-cause mortality in people with ESKD (1, 6, 7). Importantly, cardiovascular problems and frailty often manifest simultaneously in this population, and the relationship between these two conditions is likely bidirectional in nature (8).
Blood pressure (BP) dysregulation is one of the most common signs of cardiovascular dysfunction in people receiving HD and is influenced by several factors including, but not limited to, hypertension, autonomic neuropathy, HD-related fluid and electrolytes shifts, and interdialytic weight gain. Consequently, estimated dry weight and BP control are routinely monitored as part of standard dialysis care (9). One measure of BP that has gained a lot of attention in the scientific literature is BP variability (BPV). Briefly, BPV represents the physiological fluctuations of BP over time which occur as a result of a complex interplay of intrinsic cardiovascular regulatory mechanisms (e.g., aortic and carotid baroreceptors, renin-angiotensin-aldosterone system, etc.), and extrinsic factors, such as environmental stressors (e.g., heat, hydration status, etc.) (10). There are several ways of evaluating BPV that encompass measuring variations of BP in the long-term (e.g., months to years), medium term (days to weeks), short-term (minutes to hours), and very short-term (beat-to-beat fluctuations) (11, 12). Each of these methods have known strengths and limitations. Medium-to-long-term BPV assessments mainly consist of quantifying the variance (or standard deviation) of BP measurements taken over several days or months and are generally easy to implement in clinical practice. However, they offer limited information on subtle variations of BP that are attributable to the elastic properties of arteries and neurohumoral factors (13). On the other hand, beat-to-beat BPV represents the variability of the beat-to-beat BP recordings at specific frequency components, typically in the 0.02-0.50 Hz range (also known as very low to high frequencies), identified through spectral analysis (14). The variability of BP recordings at different frequency components reflects different cardio-regulatory processes, which makes beat-to-beat BPV ideal to evaluate the very short-lived physiological mechanisms regulating BP control (12).
High BPV is often regarded as a predictor of adverse cardiovascular events and all-cause mortality in ESKD (15, 16) and as a hallmark of cardiovascular, cognitive, and physical aging in geriatric populations (13). For instance, several studies have found prospective associations between long-term BPV and adverse cardiovascular events (17), cognitive decline (18), and physical frailty (19) in older adults. While the role of BPV in predicting cardiovascular risk in people living with ESKD has been extensively studied, the relationship between BPV and geriatric syndromes, such as frailty, in people living with ESKD is not well understood. Moreover, very few published reports of beat-to-beat BPV assessments in ESKD populations currently exist (20).
The objective of this study was to address these critical knowledge gaps by examining the association between very short-term (beat-to-beat) BPV and frailty in people living with ESKD and receiving hemodialysis. We hypothesized that greater BPV would be associated with higher odds of being frail in people with ESKD.
Methods
Study design, setting, and participants
Using a cross-sectional study design, we conducted a secondary analysis of BPV and frailty data collected as part of a multicenter prospective cohort study aiming to investigate the relationship between frailty, cardiovascular function, and falls in people receiving HD for ESKD (NCT02392299). The study was conducted between October 2015 and August 2018 in three dialysis units in the United Kingdom. Participants were men and women of ≥ 18 years of age, receiving HD three times a week, and able to understand written and spoken English. Exclusion criteria were: 1) clinically severe left ventricular outflow obstruction, 2) critical proximal coronary artery stenosis, 3) critical mitral stenosis, 4) critical cerebrovascular stenosis, 5) suspected or known aortic aneurysm, 6) unstable dialysis or other cardiac conditions deemed unsafe by the treating nephrologist, and 7) severe cognitive impairment. The study conformed to the ethical standards for medical research involving human participants, as laid out in the 1964 Declaration of Helsinki and its later amendments. Specifically, the study ethics were reviewed and approved by the Queen Margaret University institutional review board and by the local National Health Service research ethics committee (15/WS/0079). Before taking part in the study, all participants gave their written informed consent.
Study procedures
Participants’ demographics and clinical characteristics (e.g., age, weight, height, number of medications, years of dialysis, blood parameters) were obtained from their electronic medical records. Blood parameters were collected monthly in the dialysis units, and we took the values from the closest visit to the study assessment. The frailty and BPV data were collected as part of a single study assessment visit, which was conducted in the dialysis units on a non-HD day to minimize the confounding effects of fluid and electrolytes shifts on the BP values. The BPV assessment was conducted prior to the frailty assessment.
Frailty was evaluated using the Fried physical frailty phenotype (21). Participants who met three or more of the five components of frailty (i.e., unintentional weight loss, muscle weakness, slow gait speed, self-reported exhaustion, and low physical activity) were defined as frail, while participants meeting two components or less were defined as non-frail. Specifically, the five components of the Fried phenotype were assessed as following: 1) unintentional weight loss: loss of 10 lbs (4.5 kg) or more in the previous 12 months, ascertained by means of the participants’ electronic medical records; 2) muscle weakness: low isometric handgrip strength (using established cut-off values categorized by sex and BMI) (21); 3) slow gait speed: time to walk 15 feet (4.57 meters) higher than established cut-off values categorized by sex and height (21); 4) self-reported exhaustion: vitality score < 55 on the 36-item Short Form Survey Instrument (SF-36) (22); 5) low physical activity: low total kcal/week of physical activity using established sex-specific cut-off values, assessed through the short-form international physical activity questionnaire (IPAQ-SF) (23, 24).
Blood pressure variability (BPV) was measured using the Task Force monitor 3040i (CNSystems, Graz, Austria). Before attending the assessment visit, participants were instructed to avoid caffeinated beverages and smoking on the assessment day, to have a light meal two hours prior to the assessment, and to avoid unaccustomed physical exercise for at least 24 hours before the assessment. During the assessment, participants lay quietly awake in the supine position on an examination bed. Beat-to beat BPV was continuously recorded for 15 minutes using the Task Force monitor BP finger cuff (photoelectric plethysmography). The BP measurements were taken from the index or middle finger, using the unloading technique described by Penáz et al. (25), and were calibrated against BP measurements taken with an oscillometric BP cuff placed on the participants’ arm (free from arteriovenous fistulas). The Task Force monitor employs power spectral analysis of the beat-to-beat BP recordings using the adaptive autoregressive model proposed by Bianchi et al. (26) to calculate BPV. Specifically, the Task Force monitor calculates the total power spectral density (PSD) as well as three frequency bands for both systolic and diastolic BPV: the very low frequency (VLF, i.e., <0.04 Hz) band, the low frequency (LF, i.e., 0.04 – 0.17 Hz) band, and the high frequency (HF, i.e., 0.17 – 0.4 Hz) band. Briefly, PSD of systolic and diastolic BPV (i.e., PSD-sBP and PSD-dBP) represents an objective measure of total variability and global autonomic regulation (27). The VLF bands of systolic and diastolic BPV (i.e., VLF-sBP and VLF-dBP) are thought to be primarily influenced by myogenic vascular function, endothelial factors, and the renin-angiotensin-aldosterone system (14, 28). The LF bands of systolic and diastolic BPV (i.e., LF-sBP and LF-dBP) mainly quantify the sympathetic modulation of the sinoatrial node and vasomotor function (14, 29), while the HF bands (i.e., HF-sBP and HF-dBP) mainly reflect the parasympathetic modulation of cardiac activity (27, 30). In addition to these measures expressed as absolute values, The Task Force monitor also computes the LF and HF frequencies expressed as normalized units (i.e., LFnu-sBP and HFnu-sBP for systolic BPV, and LFnu-dBP and HFnu-dBP for diastolic BPV). Finally, the Task Force monitor also provides the LF/HF ratio of BPV (i.e., LF/HF-BPV), which is often regarded as a measure of sympathovagal balance (30).
Statistical analysis
Statistical analyses were conducted using SPSS, Version 29.0 (IBM, Inc., Armonk, NY). The Kolmogorov-Smirnov test was used to evaluate whether data were normally distributed. Demographics, clinical characteristics, and all BPV data of the study participants were summarized as mean ± standard deviation for normally distributed data, or as median and interquartile range for non-normally distributed data. Initially, we explored differences in beat-to-beat systolic and diastolic BP, as well as in all BPV parameters, between frail and non-frail participants. Independent t-tests or Mann-Whitney U tests were used, based on normal distribution assumptions. Subsequently, variables reaching a statistical significance level of p ≤ 0.1 were entered in a logistic regression model adjusted for age and sex with frailty status (i.e., “yes” or “no”) as the dependent variable. In addition, as a sensitivity analysis, we conducted another stage of multivariable analysis by entering diabetes as a covariate into the same logistic regression model (Model 2). This was done to control for the confounding effects of diabetic nephropathy on measures of BPV. A significance level of p < 0.05 was used for the statistical interpretation of the study findings.
Results
Overall, 76 people receiving HD for ESKD participated in this observational study. However, the data from seven participants were excluded, as their continuous BP measurements were unusable due to inadequate blood flow to the fingers. Therefore, the data of 69 participants were included in the final analysis. The demographic and clinical characteristics of the study participants are fully summarized in Table 1. Twenty-six (37.7%) participants met the Fried physical frailty criteria and were classified as frail, while the remaining 43 participants were classified as non-frail. Compared to the non-frail, frail participants were older (66.8 ± 10.0 years vs. 57.7 ± 15.2 years, p = 0.004), had a higher number of prescribed medications (13.5 [6.5] vs. 11.0 [4.0], p = 0.037), and lower albumin (36.0 [5.3] g/L vs. 38.0 [4.0] g/L, p = 0.005) and creatinine (547.5 ± 131.4 umol/L vs. 664.4 ± 138.5 umol/L, p = 0.001) levels.
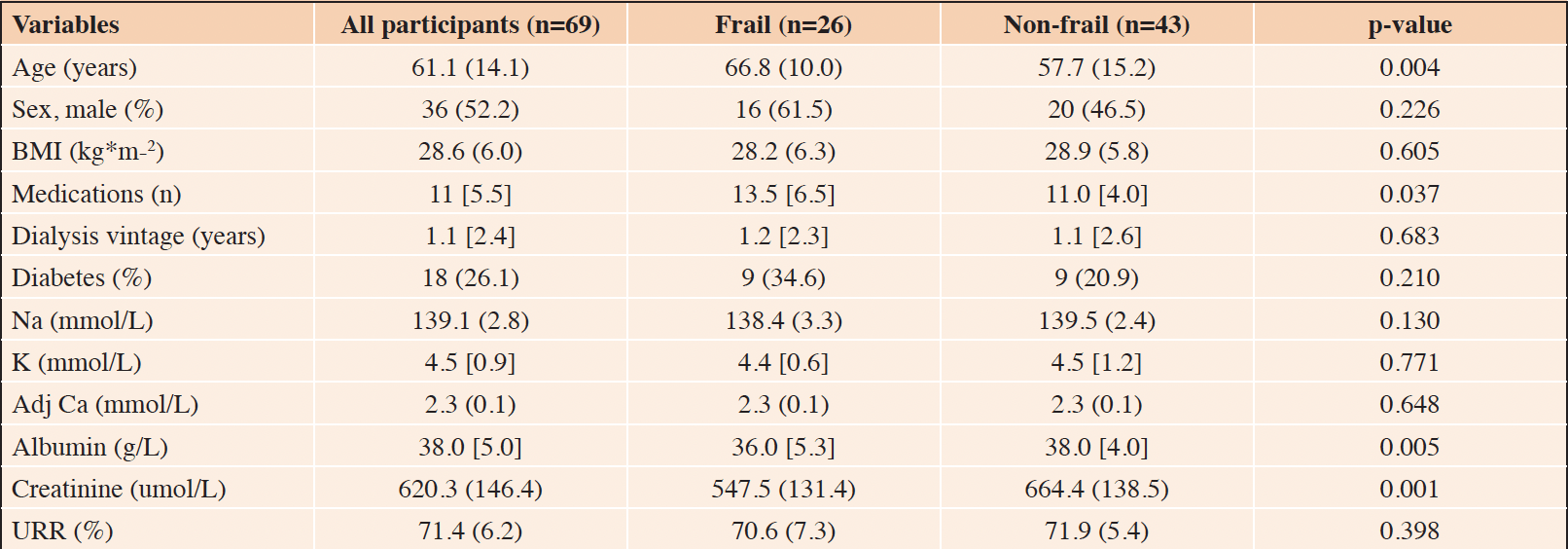
Table 1. Demographic and clinical characteristics of the study participants: results are summarized as median [interquartile range] or as mean (standard deviation)
Legend: BMI: body mass index; Na: sodium; K: potassium; Adj Ca: adjusted calcium; URR: urea reduction ratio.
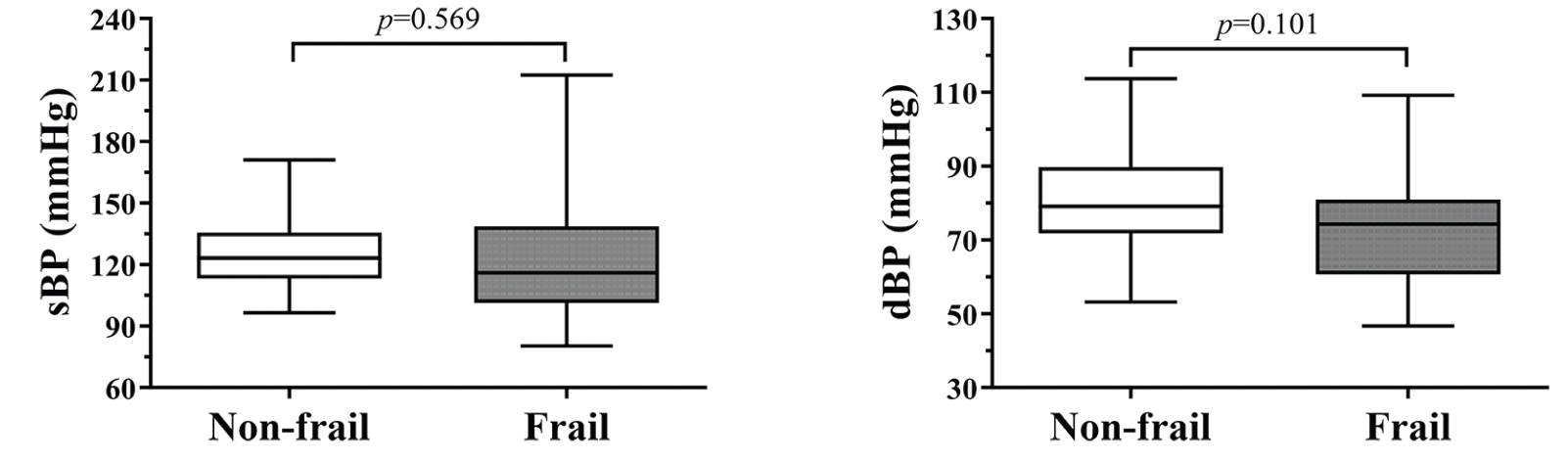
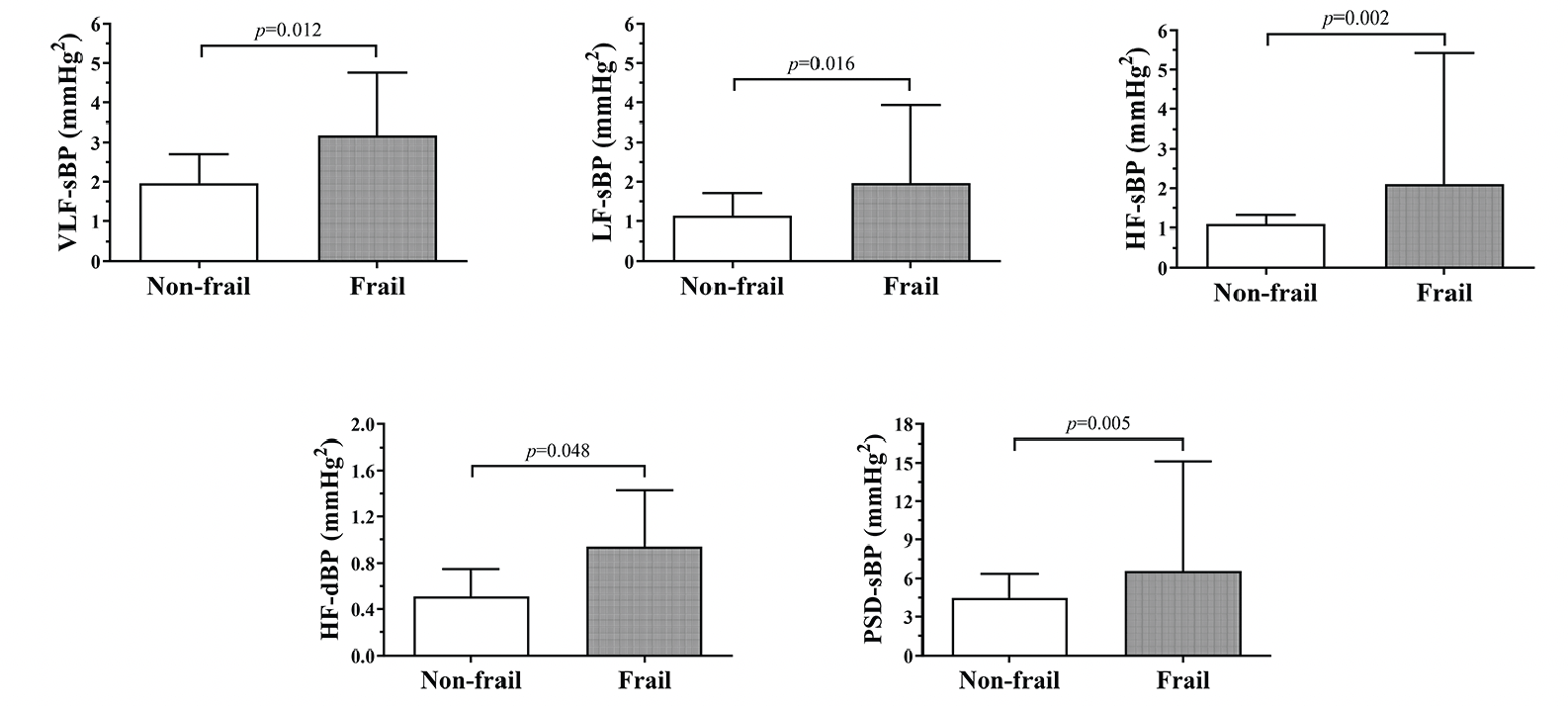
The mean beat-to-beat systolic and diastolic BP values in the whole study population were 124.0 ± 22.4 mmHg and 77.8 ± 14.9 mmHg, respectively. No differences between frail and non-frail participants were detected (Figure 1). The BPV data are fully summarized in Table 2. The Mann-Whitney U tests identified several statistically significant differences in systolic BPV between frail and non-frail participants. Particularly, frail individuals had higher VLF-sBP, LF-sBP, HF-sBP, and PSD-sBP compared to their non-frail counterparts. In addition, frail participants had higher HF-dBP than the non-frail (Figure 2).
Legend: sBP: systolic blood pressure; dBP: diastolic blood pressure.
Legend: sBP: systolic blood pressure; dBP: diastolic blood pressure; VLF: very low frequency band; LF: low frequency band; HF: high frequency band; PSD: power spectral density.
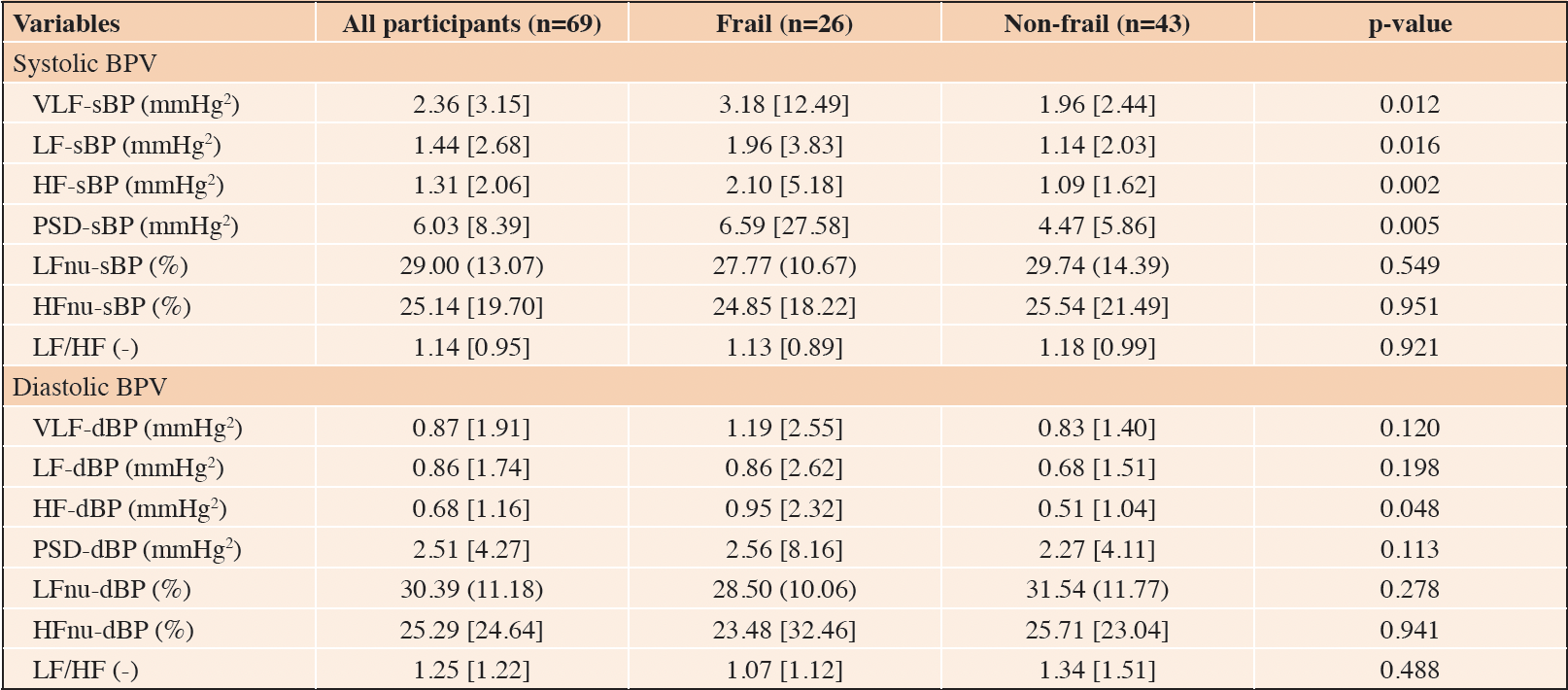
Table 2. Differences in measures of BPV between frail and non-frail participants: results are summarized as median [interquartile range] or as mean (standard deviation)
Legend: sBP: systolic blood pressure; dBP: diastolic blood pressure; VLF: very low frequency band; LF: low frequency band; HF: high frequency band; PSD: power spectral density; nu: normalized units; LF/HF: low frequency/high frequency ratio.
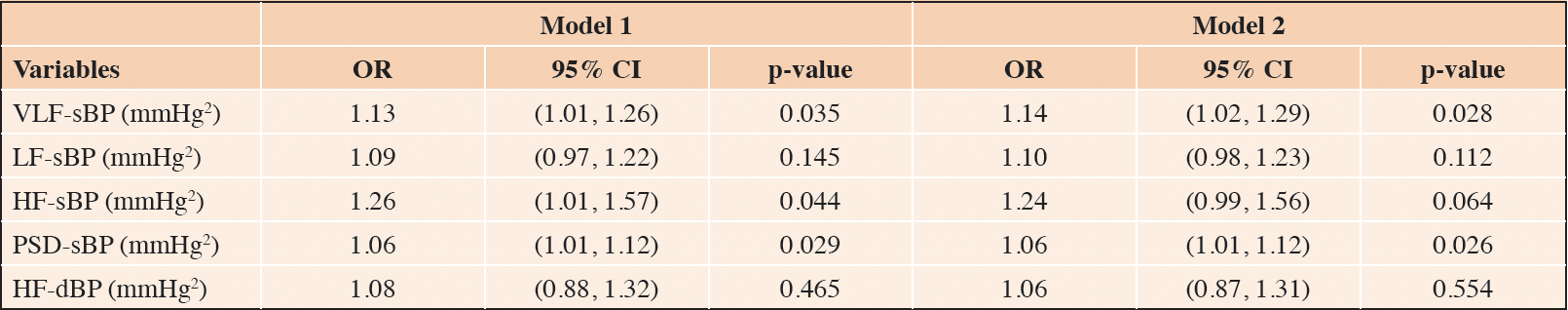
The age- and sex-adjusted logistic regression analyses, summarized in Table 3, revealed that only VLF-sBP (odds ratio [OR] = 1.13, 95% confidence interval [CI]: 1.01 – 1.26, p = 0.035), HF-sBP (OR = 1.26, 95% CI: 1.01 – 1.57, p = 0.044), and PSD-sBP (OR = 1.06, 95% CI: 1.01 – 1.12, p = 0.029) were associated with increased odds of being frail. In the sensitivity logistic regression analysis (i.e., Model 2: adjusted for age, sex, and diabetic status) only VLF-sBP (OR = 1.14, 95% CI: 1.02 – 1.29, p = 0.028) and PSD-sBP (OR = 1.06, 95% CI: 1.01 – 1.12, p = 0.026) remained significantly associated with frailty.
Legend: OR: odds ratio; CI: confidence interval; sBP: systolic blood pressure; dBP: diastolic blood pressure; VLF: very low frequency band; LF: low frequency band; HF: high frequency band; PSD: power spectral density; Model 1 is adjusted for age and sex; Model 2 is adjusted for age, sex, and diabetes.
Discussion
In this study, we aimed to examine the association between very short-term (beat-to-beat) BPV and frailty in people receiving hemodialysis for ESKD. Our analysis revealed that frail ESKD participants had higher systolic BPV in the VLF, LF, and HF bands, as well as in total PSD. The relationship between diastolic BPV and frailty was less clear, as we identified only one statistically significant difference between frail and non-frail participants in the HF band. Furthermore, after adjustment for age and sex, only systolic BPV measures were significantly associated with frailty status in logistic regression analyses. The study findings thus suggest that greater systolic, but not diastolic, BPV is associated with higher odds of being frail in people living with ESKD. In addition, no differences in beat-to-beat systolic and diastolic BP values were detected between frail and non-frail individuals. This observation provides indirect evidence that BPV measurements may be more indicative of frailty status or risk than basic BP assessments in this clinical population.
The relationship between BPV and frailty is likely complex and bidirectional in nature. Higher BPV is described as an epiphenomenon of cardiovascular aging, which is also implicated in the etiology of frailty (13). However, frailty can further aggravate cardiovascular function and outcomes through muscle disuse due to the physical limitations of frail individuals (31). Although the cross-sectional design used in our investigation does not allow us to draw any inference on the presumed directionality of the observed association between BPV and frailty, the logistic regression analyses revealed that higher systolic BPV was associated with frailty status independent of chronological age (Table 3). This finding highlights the possibility that higher BPV may represent a hallmark of accelerated aging in people with ESKD. In addition, the observation that systolic, but not diastolic, BPV was associated with frailty is strongly aligned with findings from previous studies conducted in non-CKD populations (19, 32). For instance, Rouch et al. (33) examined the relationship between visit-to-visit BPV and incident frailty in a large cohort of community-dwelling older adults (n = 1,394), and they concluded that greater systolic, but not diastolic, BPV was associated with a higher risk of developing frailty over five years. As the authors pointed out, this finding may reflect the notion that, compared to diastolic BP, the systolic BP response tends to be a better predictor of adverse outcomes associated with aging (and CKD), such as cardiovascular events and all-cause mortality (34). In addition, Zhu et al. (32) examined the relationship between BPV, derived from 24-hour automated BP monitoring, and frailty in 242 hypertensive patients (mean age = 73.0 ± 9.4 years). The study results mirrored those of Rouch et al. (33), as greater systolic, but not diastolic, BPV was cross-sectionally associated with frailty in their sample. The authors postulated that this finding may be primarily ascribable to the impaired baroreceptor modulation of the sympathetic drive that is often observed in aging individuals (32). However, the link between systolic BPV and frailty is not fully understood, and more research would be needed to explore this hypothesis.
People with ESKD have multiple physiologic alterations of cardiovascular function that may reflect abnormal BPV. For instance, arterial stiffness, due to vascular calcification and/or endothelial dysfunction, and baroreflex impairment are very common in ESKD (35, 36) and are thought to contribute to higher BPV (13). In addition, BPV changes are also observed with diabetic neuropathy and hypertension, both of which are highly prevalent in people living with ESKD (37, 38). Relatedly, a population-based cohort study by Wan et al. (39) has recently shown that higher systolic BPV is associated with a greater risk of developing CKD in > 200,000 people living with hypertension (39% higher risk for each 10 mmHg increase in the standard deviation of systolic BP). This finding is noteworthy because it provides evidence that alterations in BPV may be implicated in the etiology of CKD and, by extension, in the etiology of CKD-related frailty. Consequently, high BPV can be reasonably expected not only in ESKD but also at the earlier stages of CKD, which underscores the importance of timely BPV evaluations. In people receiving HD, the hemodynamic instability due to inadequate fluid and electrolyte shifts is also likely to further affect BPV compared to individuals with more preserved kidney function (40).
The spectral analysis of the BP recordings revealed that frail participants had higher systolic BPV in several frequency bands (i.e., VLF, LF, HF) as well as in total PSD compared to non-frail participants (Figure 2). These findings suggest that frail individuals had lower global autonomic function, with involvement of both the sympathetic and parasympathetic nervous systems (27). Interestingly, even after adjustment for age, sex, and diabetes, VLF-sBP and PSD-sBP remained significantly associated with frailty (Table 3). While the LF and HF bands quantify two central components of BP regulation, namely the sympathetic and parasympathetic modulation of cardiac activity, respectively, the VLF band reflects the contribution of more peripherical mechanisms (29). Specifically, VLF-sBP is thought to be influenced by myogenic vascular function, the endothelium, and the renin-angiotensin-aldosterone system (28). This suggests that frail individuals may have diminished endothelial function and lower elastic properties of the vasculature compared to the non-frail. Endothelial cellular senescence and the lower bioavailability of nitric oxide have been linked to alterations of BPV and are plausibly implicated in the etiology of frailty (13). This observation may also reflect the vascular calcification that is often observed in people with ESKD and the role it may play in frailty progression.
Reducing BPV in people living with ESKD, as well as with earlier stages of CKD and/or hypertension, likely represents an important therapeutic goal (41). Overall, all antihypertensive medications have the potential to reduce BP fluctuations in both the short- and long-term (42). However, calcium channel blockers (often used in combination with diuretics) seem to be the most effective drug class for reducing BPV due to their ability to improve arterial compliance and measures of autonomic function (43). For instance, a 2014 meta-analysis concluded that amlodipine was superior to other classes of antihypertensive medications, with a treatment difference (standard error) of -1.23 (0.46; p = 0.008) mmHg using the standard deviation of BP and -0.86 (0.31; p = 0.005) using the coefficient of variation vs. the other active comparators (44). Calcium channel blockers and other hypertensive medications are commonly used in people with CKD, and, by the time a patient reaches the stage of requiring HD for ESKD, being prescribed antihypertensive medications is extremely common (9). This brings to light the need for additional therapeutic strategies to minimize BPV in the ESKD population. Non-pharmacological interventions such as diet and exercise are two highly recommended strategies for achieving optimal BP control in people receiving HD (45). To date, no studies have directly examined the effects of exercise interventions on measures of BPV in people with ESKD to the best of our knowledge. However, a recent meta-analysis by Lin et al. (46) concluded that, following exercise training, systolic BPV was significantly improved in people living with hypertension (effect size = -0.68, 95% CI: -1.18 to -0.18, I2 64%). Notably, the study findings also underscore the importance of assessing BPV in ESKD populations. Although the feasibility of performing beat-to-beat BP monitoring may be limited in clinical settings, standard oscillometric BP measurements are routinely recorded during hospital-based dialysis. This highlights the perhaps still untapped opportunity to generate BPV metrics (e.g., coefficients of variation and standard deviation of BP readings) at virtually no cost that could be used to monitor important therapeutic goals, such as adherence to antihypertensive therapy (12), and/or to gauge cardiovascular and frailty risk over time.
The current study has several strengths and limitations. For instance, this is, to the best of our knowledge, the first investigation to show an association between higher BPV and frailty in an ESKD population. Additionally, we used continuous, non-invasive BP monitoring to evaluate BPV. The beat-to-beat assessment of BPV allowed us to gain more insights on the relationship between the very short-lived physiological mechanisms regulating BP control and frailty in the studied sample. On the other hand, the cross-sectional study design limited any inferences that could be drawn about causality. In this respect, more studies with a prospective design would be needed to elucidate the relationship between BPV and frailty in people living with CKD/ESKD. In addition, we should acknowledge that the sample size was relatively small (n=69). This may have resulted in higher chances of committing a type-II error. For example, the lack of association between diastolic BPV and frailty may be explained by insufficient power to detect differences between frail and non-frail participants. Finally, the lack of an age- and sex-matched control group without ESKD may also be construed as a study limitation. Particularly, the absence of “reference” values for the BPV measures examined makes the clinical interpretation of the study findings more challenging.
Conclusions
Higher systolic BPV is associated with frailty in people receiving hemodialysis for ESKD. Beat-to-beat assessments of BPV through continuous, non-invasive blood pressure monitoring may be useful in evaluating not only cardiovascular risk but also frailty risk in CKD populations. More studies with a prospective design are needed to elucidate the relationship between BPV and frailty in the context of ESKD, as well as to demonstrate the longitudinal association between higher BPV and patient-reported and clinical outcomes, such as impaired cognition, hospitalizations, and mortality in people with advanced CKD.
Conflicts of Interest: The authors declare they have no conflicts of interest.
Funding: This work is supported by funding from a British Kidney Patient Association-British Renal Society joint grant; grant number: 16-003.
Ethics declarations: The study conformed to the ethical standards for medical research involving human participants, as laid out in the 1964 Declaration of Helsinki and its later amendments. The study ethics were reviewed and approved by the Queen Margaret University institutional review board and by the local National Health Service research ethics committee (15/WS/0079). Before taking part in the study, all participants gave their written informed consent.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Evans M, Lewis RD, Morgan AR, Whyte MB, Hanif W, Bain SC, et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv Ther. 2022;39(1):33-43. doi: 10.1007/s12325-021-01927-z.
2. Hannan M, Chen J, Hsu J, Zhang X, Saunders MR, Brown J, et al. Frailty and Cardiovascular Outcomes in Adults With CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2024;83(2):208-15. doi: 10.1053/j.ajkd.2023.06.009.
3. Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143(11):1157-72. doi: 10.1161/CIRCULATIONAHA.120.050686.
4. Kojima G. Prevalence of frailty in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49(11):1989-97. doi: 10.1007/s11255-017-1547-5.
5. Zhao Y, Liu Q, Ji J. The prevalence of frailty in patients on hemodialysis: a systematic review and meta-analysis. Int Urol Nephrol. 2020;52(1):115-20. doi: 10.1007/s11255-019-02310-2.
6. Zanotto T, Mercer TH, van der Linden ML, Rush R, Traynor JP, Petrie CJ, et al. The relative importance of frailty, physical and cardiovascular function as exercise-modifiable predictors of falls in haemodialysis patients: a prospective cohort study. BMC Nephrol. 2020;21(1):99. doi: 10.1186/s12882-020-01759-z.
7. Tonelli M, Wiebe N, Gill JS, Bello AK, Hemmelgarn BR, Chan CT, et al. Frailty and Clinical Outcomes in Patients Treated With Hemodialysis: A Prospective Cohort Study. Kidney Med. 2023;5(8):100684. doi: 10.1016/j.xkme.2023.100684.
8. Adenwalla SF, Billany RE, March DS, Gulsin GS, Young HML, Highton P, et al. The cardiovascular determinants of physical function in patients with end-stage kidney disease on haemodialysis. Int J Cardiovasc Imaging. 2021;37(4):1405-14. doi: 10.1007/s10554-020-02112-z.
9. Miskulin DC, Weiner DE. Blood Pressure Management in Hemodialysis Patients: What We Know And What Questions Remain. Semin Dial. 2017;30(3):203-12. doi: 10.1111/sdi.12586.
10. Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich). 2018;20(7):1133-7. doi: 10.1111/jch.13304.
11. Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol. 2022;19(10):643-54. doi: 10.1038/s41569-022-00690-0.
12. Sheikh AB, Sobotka PA, Garg I, Dunn JP, Minhas AMK, Shandhi MMH, et al. Blood Pressure Variability in Clinical Practice: Past, Present and the Future. J Am Heart Assoc. 2023;12(9):e029297. doi: 10.1161/JAHA.122.029297.
13. Bencivenga L, De Souto Barreto P, Rolland Y, Hanon O, Vidal JS, Cestac P, et al. Blood pressure variability: A potential marker of aging. Ageing Res Rev. 2022;80:101677. doi: 10.1016/j.arr.2022.101677.
14. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25(6):1276-86. doi: 10.1161/01.hyp.25.6.1276.
15. Tozawa M, Iseki K, Yoshi S, Fukiyama K. Blood pressure variability as an adverse prognostic risk factor in end-stage renal disease. Nephrol Dial Transplant. 1999;14(8):1976-81. doi: 10.1093/ndt/14.8.1976.
16. Chang TI, Tabada GH, Yang J, Tan TC, Go AS. Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. J Hypertens. 2016;34(2):244-52. doi: 10.1097/HJH.0000000000000779.
17. Ernst ME, Chowdhury EK, Beilin LJ, Margolis KL, Nelson MR, Wolfe R, et al. Long-Term Blood Pressure Variability and Risk of Cardiovascular Disease Events Among Community-Dwelling Elderly. Hypertension. 2020;76(6):1945-52. doi: 10.1161/HYPERTENSIONAHA.120.16209.
18. Ernst ME, Ryan J, Chowdhury EK, Margolis KL, Beilin LJ, Reid CM, et al. Long-Term Blood Pressure Variability and Risk of Cognitive Decline and Dementia Among Older Adults. J Am Heart Assoc. 2021;10(13):e019613. doi: 10.1161/JAHA.120.019613.
19. Fravel MA, Ernst ME, Woods RL, Beilin L, Zhou Z, Orchard SG, et al. Long-term blood pressure variability and frailty risk in older adults. J Hypertens. 2024;42(2):244-51. doi: 10.1097/HJH.0000000000003599.
20. Flythe JE, Brunelli SM. Blood pressure variability among chronic dialysis patients: recent advances in knowledge. Curr Opin Nephrol Hypertens. 2015;24(2):163-9. doi: 10.1097/MNH.0000000000000107.
21. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56. doi: 10.1093/gerona/56.3.m146.
22. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960-7. doi: 10.1681/ASN.2007020221.
23. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-95. doi: 10.1249/01.MSS.0000078924.61453.FB.
24. Zanotto T, Mercer TH, van der Linden ML, Traynor JP, Koufaki P. Use of a wearable accelerometer to evaluate physical frailty in people receiving haemodialysis. BMC Nephrol. 2023;24(1):82. doi: 10.1186/s12882-023-03143-z.
25. Penaz J, Voigt A, Teichmann W. [Contribution to the continuous indirect blood pressure measurement]. Z Gesamte Inn Med. 1976;31(24):1030-3.
26. Bianchi AM, Mainardi LT, Meloni C, Chierchia S, Cerutti S. Continuous monitoring of the sympatho-vagal balance through spectral analysis. IEEE Eng Med Biol Mag. 1997;16(5):64-73. doi: 10.1109/51.620497.
27. Frith J, Zalewski P, Klawe JJ, Pairman J, Bitner A, Tafil-Klawe M, et al. Impaired blood pressure variability in chronic fatigue syndrome–a potential biomarker. QJM. 2012;105(9):831-8. doi: 10.1093/qjmed/hcs085.
28. Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol. 2007;34(4):362-8. doi: 10.1111/j.1440-1681.2007.04588.x.
29. Tian G, Xiong L, Leung H, Soo Y, Leung T, Wong LK. Beat-to-beat blood pressure variability and heart rate variability in relation to autonomic dysregulation in patients with acute mild-moderate ischemic stroke. J Clin Neurosci. 2019;64:187-93. doi: 10.1016/j.jocn.2019.03.003.
30. Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249(4 Pt 2):H867-75. doi: 10.1152/ajpheart.1985.249.4.H867.
31. Talha KM, Greene SJ, Butler J, Khan MS. Frailty and Its Implications in Heart Failure with Reduced Ejection Fraction: Impact on Prognosis and Treatment. Cardiol Clin. 2023;41(4):525-36. doi: 10.1016/j.ccl.2023.06.002.
32. Zhu Y, Chen X, Geng S, Li Q, Yuan H, Zhou X, et al. Association between ambulatory blood pressure variability and frailty among older hypertensive patients. J Clin Hypertens (Greenwich). 2020;22(9):1703-12. doi: 10.1111/jch.13986.
33. Rouch L, De Souto Barreto P, Hanon O, Vidal JS, Amar J, Andrieu S, et al. Visit-to-Visit Blood Pressure Variability and Incident Frailty in Older Adults. J Gerontol A Biol Sci Med Sci. 2021;76(8):1369-75. doi: 10.1093/gerona/glab112.
34. Lee JY, Park JT, Joo YS, Lee C, Yun HR, Chang TI, et al. Association of blood pressure with cardiovascular outcome and mortality: results from the KNOW-CKD study. Nephrol Dial Transplant. 2022;37(9):1722-30. doi: 10.1093/ndt/gfab257.
35. Gusbeth-Tatomir P, Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. Kidney Blood Press Res. 2007;30(2):97-107. doi: 10.1159/000100905.
36. Zanotto T, Mercer TH, van der Linden ML, Traynor JP, Petrie CJ, Doyle A, et al. Baroreflex function, haemodynamic responses to an orthostatic challenge, and falls in haemodialysis patients. PLoS One. 2018;13(12):e0208127. doi: 10.1371/journal.pone.0208127.
37. Spallone V. Blood Pressure Variability and Autonomic Dysfunction. Curr Diab Rep. 2018;18(12):137. doi: 10.1007/s11892-018-1108-z.
38. Nardin C, Rattazzi M, Pauletto P. Blood Pressure Variability and Therapeutic Implications in Hypertension and Cardiovascular Diseases. High Blood Press Cardiovasc Prev. 2019;26(5):353-9. doi: 10.1007/s40292-019-00339-z.
39. Wan EYF, Yu EYT, Chin WY, Fong DYT, Choi EPH, Lam CLK. Association of visit-to-visit variability of systolic blood pressure with cardiovascular disease, chronic kidney disease and mortality in patients with hypertension. J Hypertens. 2020;38(5):943-53. doi: 10.1097/HJH.0000000000002347.
40. Jin Y, Huang X, Zhang C, Xie J, Ren H. Impact of fluid overload on blood pressure variability in patients on peritoneal dialysis. Ren Fail. 2022;44(1):2066-72. doi: 10.1080/0886022X.2022.2148535.
41. Yang J, Huang J, Yu B, Zhang Q, Zhang S, Wu L, et al. Long-term predialysis blood pressure variability and outcomes in hemodialysis patients. J Clin Hypertens (Greenwich). 2022;24(2):148-55. doi: 10.1111/jch.14398.
42. Eguchi K. Effects of Antihypertensive Therapy on Blood Pressure Variability. Curr Hypertens Rep. 2016;18(10):75. doi: 10.1007/s11906-016-0680-3.
43. Wang JG, Palmer BF, Vogel Anderson K, Sever P. Amlodipine in the current management of hypertension. J Clin Hypertens (Greenwich). 2023;25(9):801-7. doi: 10.1111/jch.14709.
44. Wang JG, Yan P, Jeffers BW. Effects of amlodipine and other classes of antihypertensive drugs on long-term blood pressure variability: evidence from randomized controlled trials. J Am Soc Hypertens. 2014;8(5):340-9. doi: 10.1016/j.jash.2014.02.004.
45. Georgianos PI, Agarwal R. Blood pressure control in conventional hemodialysis. Semin Dial. 2018;31(6):557-62. doi: 10.1111/sdi.12741.
46. Lin M, Lin Y, Li Y, Lin X. Effect of exercise training on blood pressure variability in adults: A systematic review and meta-analysis. PLoS One. 2023;18(10):e0292020. doi: 10.1371/journal.pone.0292020.
The Author(s) 2024